INDICATIONS AND USAGE Clapanb (Clanib) is a poly(ADPribose) polymerase (PARP) inhibitor indicated as monotherapy in patients with deletenous o suspected deleterious germ line BRCA mutated (as detected by an FDA-approved test) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy, DOSAGE AND ADMINISTRATION The recommended dose of Claparib (Canibis 400 mg (eight 50 mg capsicles) taken twice daily, for a total daly dose of 800 mg. Continue treatment until disease progression or unaccept- able toxicity. a patient misses a dose of Claparib (anb) instruct patient to take their nest dose at its scheduled time. Swallow capsule whole. Do not chew, dissolve or open capsule, Do not take capsules which appear deformed or show evidence of leakage. Dose Adjustments for Adverse Reactions To manage adverse reactions, consider dose interruption of treatment or dose reduction, The recommended dose reduction is to 200 mg four 50) mg capsules) taken twice daily, for a total daily dose of 400 mg. I a further final dose reduction is required, then reduce to 100 mg two 50 mg capsules) taken twice daily for a total daily dose of 200 mg. Dose Modifications for Use with CYP3A Inhibitors Avoid concomitant use of strong and moderate CYP3A inhibitors and consider alternative agents with less CYP3A inhibition. If the inhibitor cannot be avoided, reduce the Disparo (Clanib) dose la 150 mg (three 50 mg capsules) taken twice daily for a strang CYP3A inhibitor or 200 mg (four 50 mg capsules) taken twe daily for a moderate CYP3A Inhibitor. Dose Modifications for Patients with Renal Impairment Patients with mild renal impairment (CLet 51-80 mL/min as estimated by Cockcroft-Gault) do not require an adjustment in Claparib (Olanib) dosing. In patients with moderate real impairment (Cler 31-50 mL/min) the recommended dose reduction is to 300 mg (six 50 mg capsubs) taken twice daily, for a total daily dose of 600 mg. The pharmacokinetics of Claparib (Clanib) have not been evaluated in patients with severe renal impairment or end-stage renal disease (CLE 30 min

Tyrosine kinase inhibitor
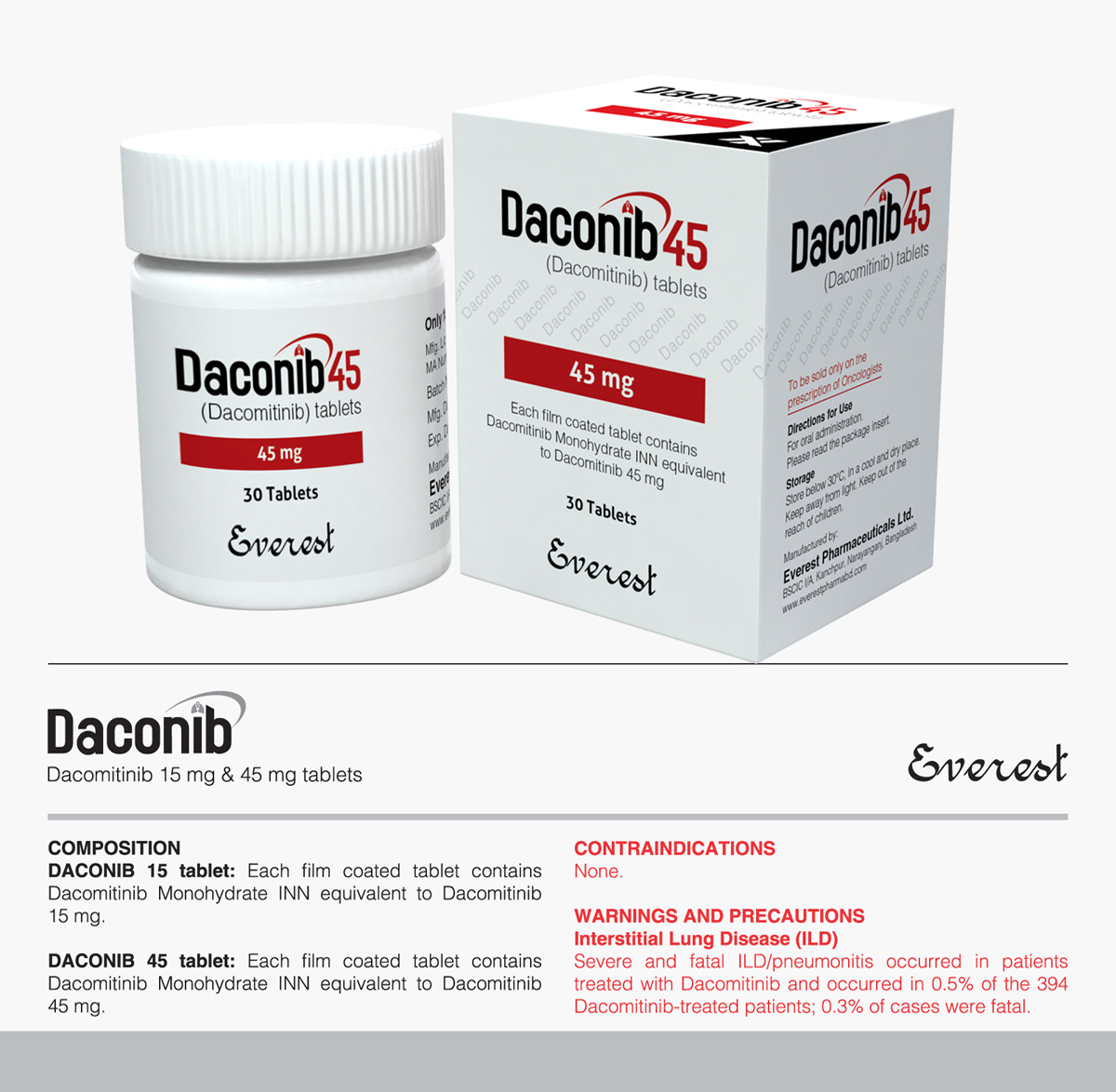
Tyrosin Kinase Inhibitor (TKI)
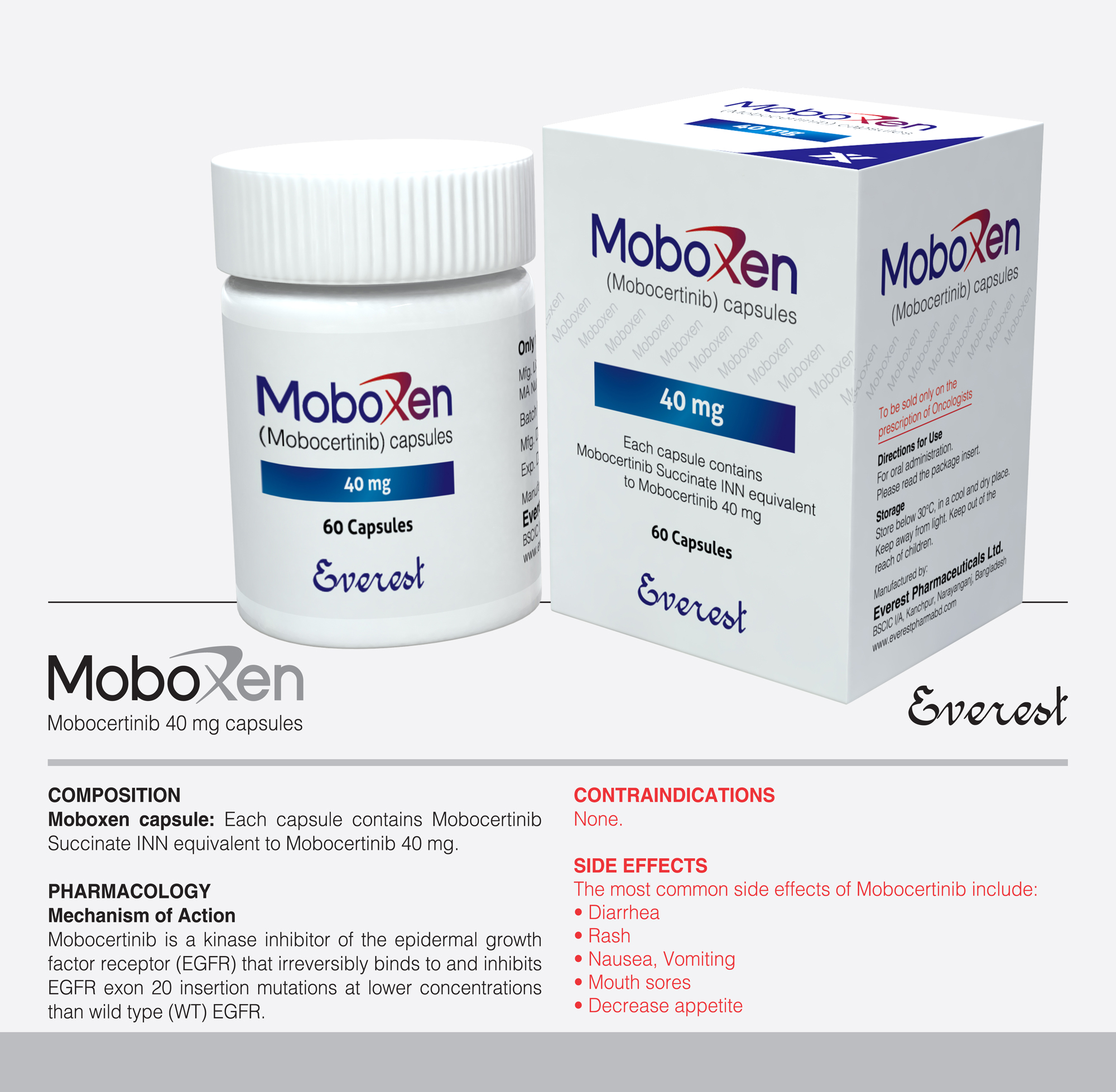
Tyrosine kinase inhibitor
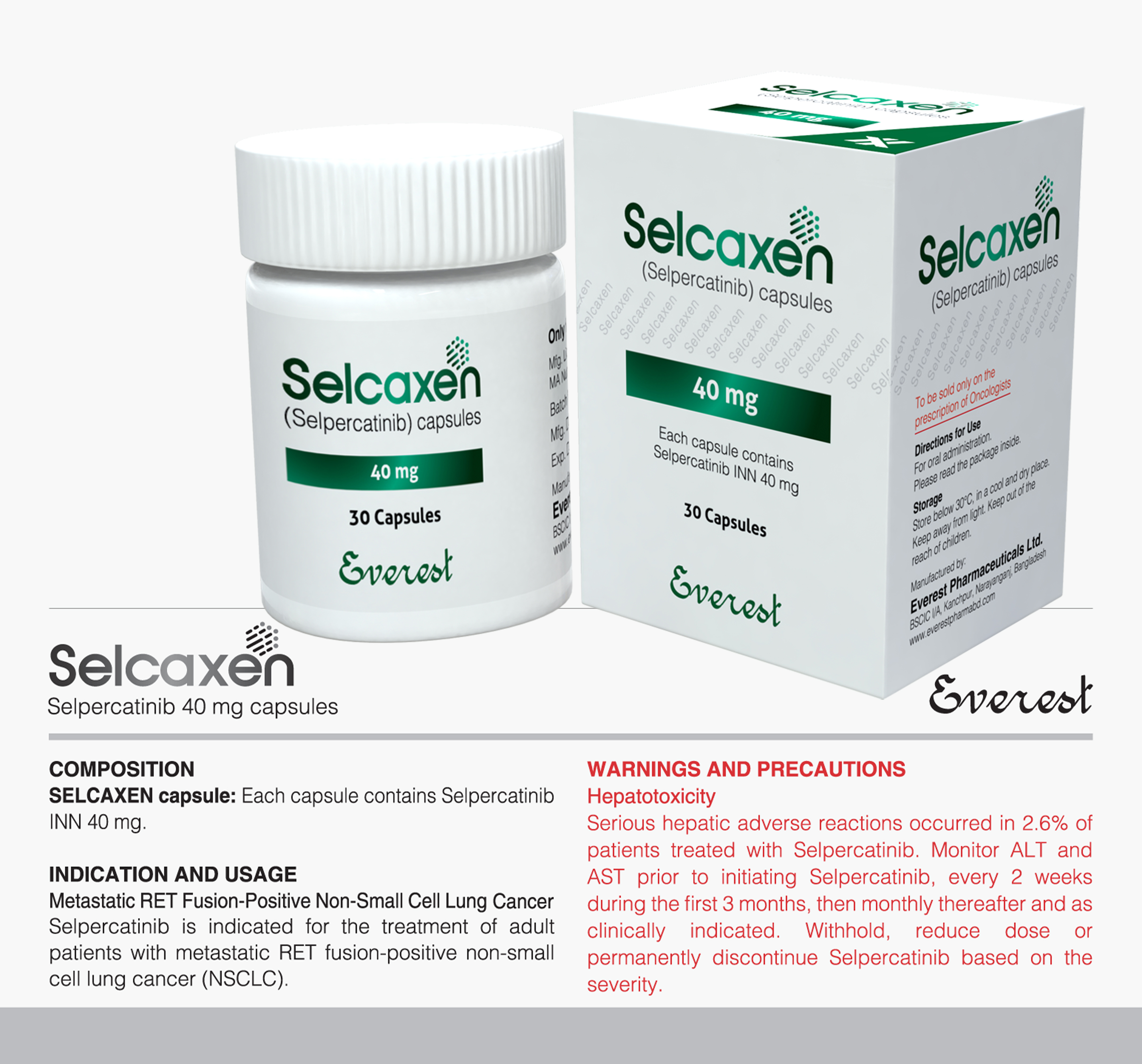
Tyrosine kinase inhibitor

Tyrosine kinase inhibitor

Mezoxia
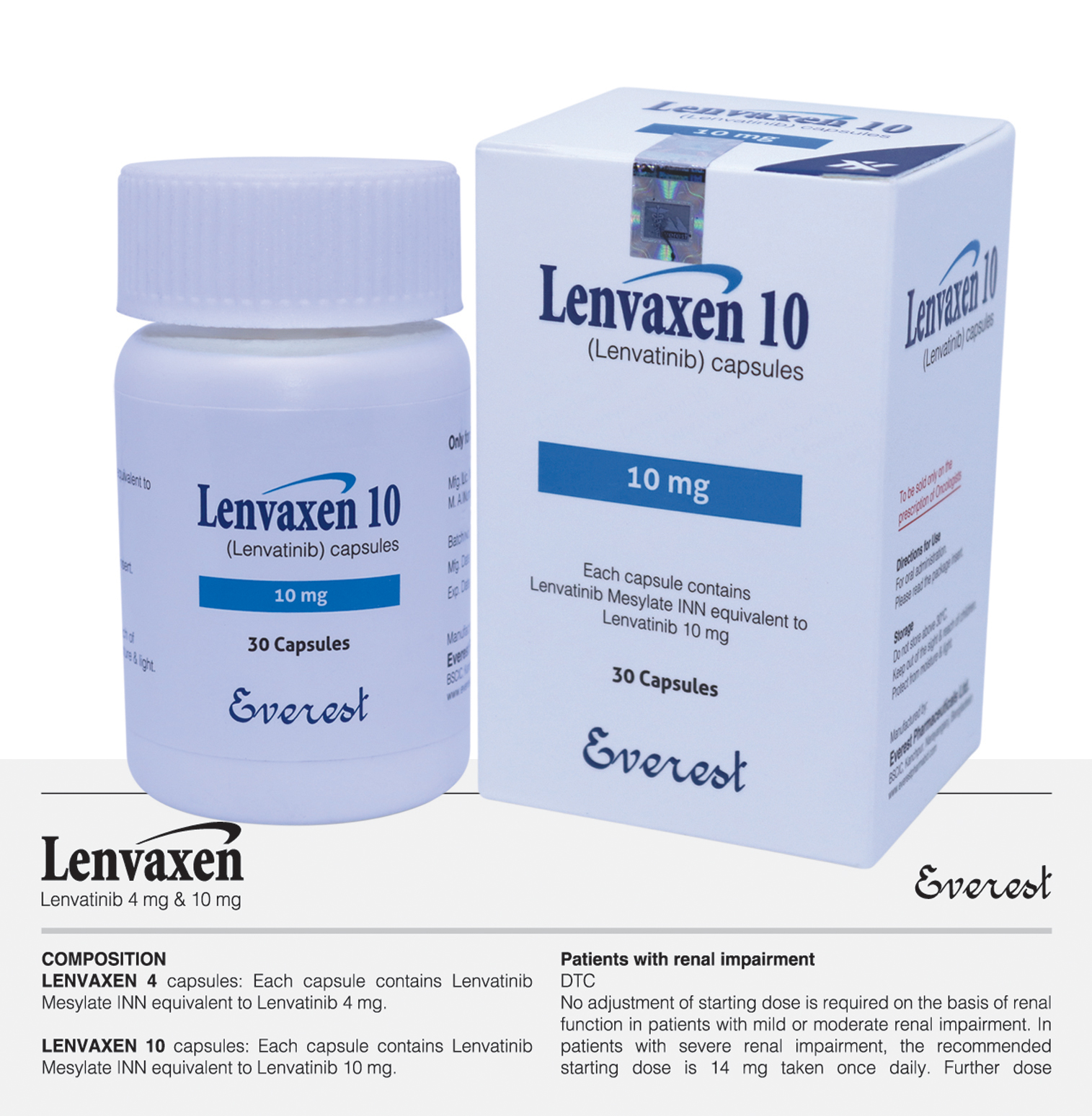
Tyrosine kinase inhibitor

KRAS inhibitors
Tyrosin Kinase Inhibitor (TKI)
B-cell lymphoma-2 (BCL-2) inhibitors