INDICATIONS AND USAGE Mobocertinib is a kinase inhibitor indicated for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
DOSAGE AND ADMINISTRATION Patient Selection Select patients with locally advanced or metastatic NSCLC for treatment with Mobocertinib based on the presence of EGFR exon 20 insertion mutations. Recommended Dosage The recommended dosage of Mobocertinib is 160 mg orally once daily until disease progression or unacceptable toxicity. Take Mobocertinib with or without food, at the same time each day. Swallow Mobocertinib capsules whole. Do not open, chew or dissolve the contents of the capsules. If a dose is missed by more than 6 hours, skip the dose and take the next dose the following day at its regularly scheduled time. If a dose is vomited, do not take an additional dose

Tyrosine kinase inhibitor
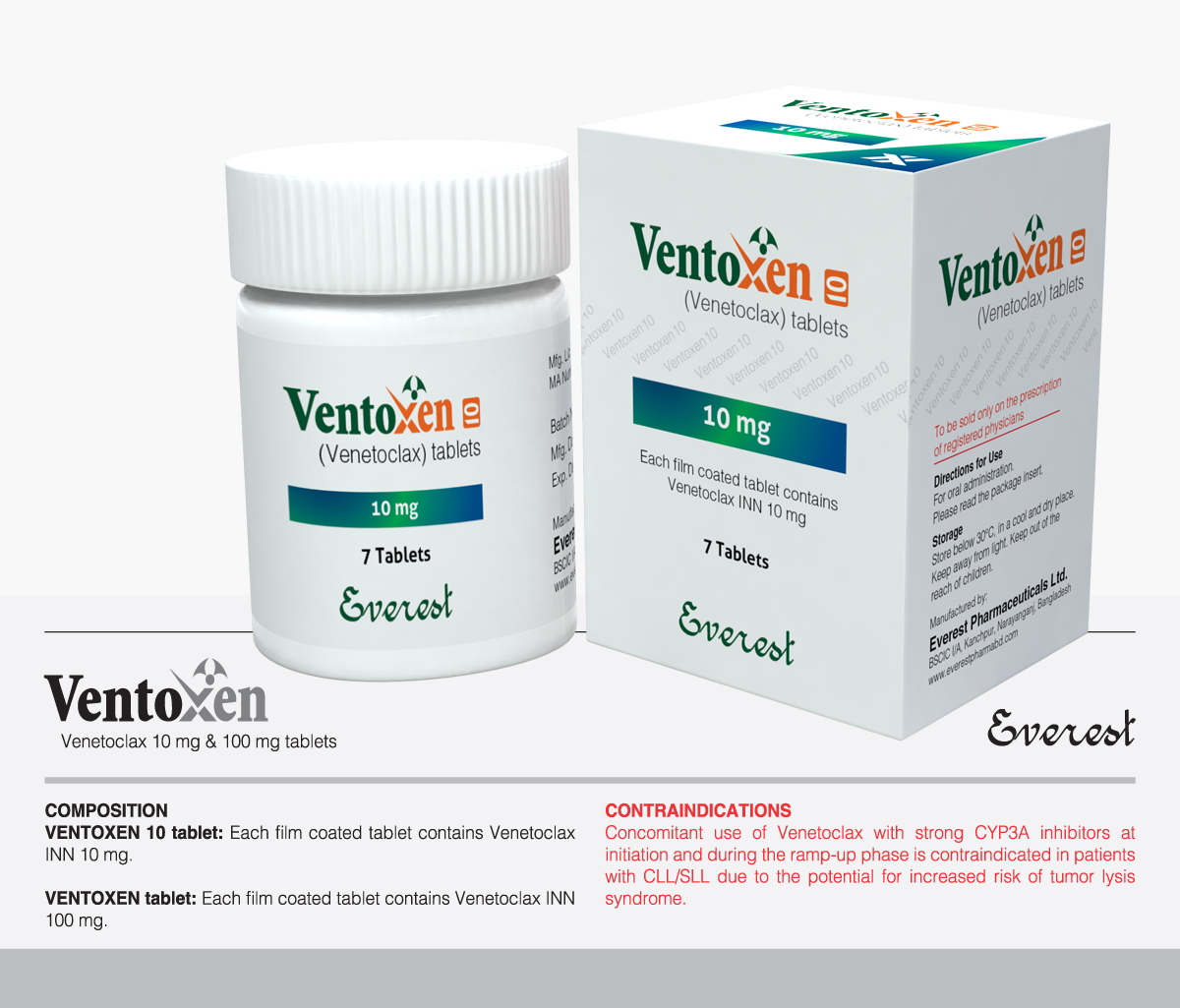
B-cell lymphoma-2 (BCL-2) inhibitors

Tyrosine kinase inhibitor

KRAS inhibitors
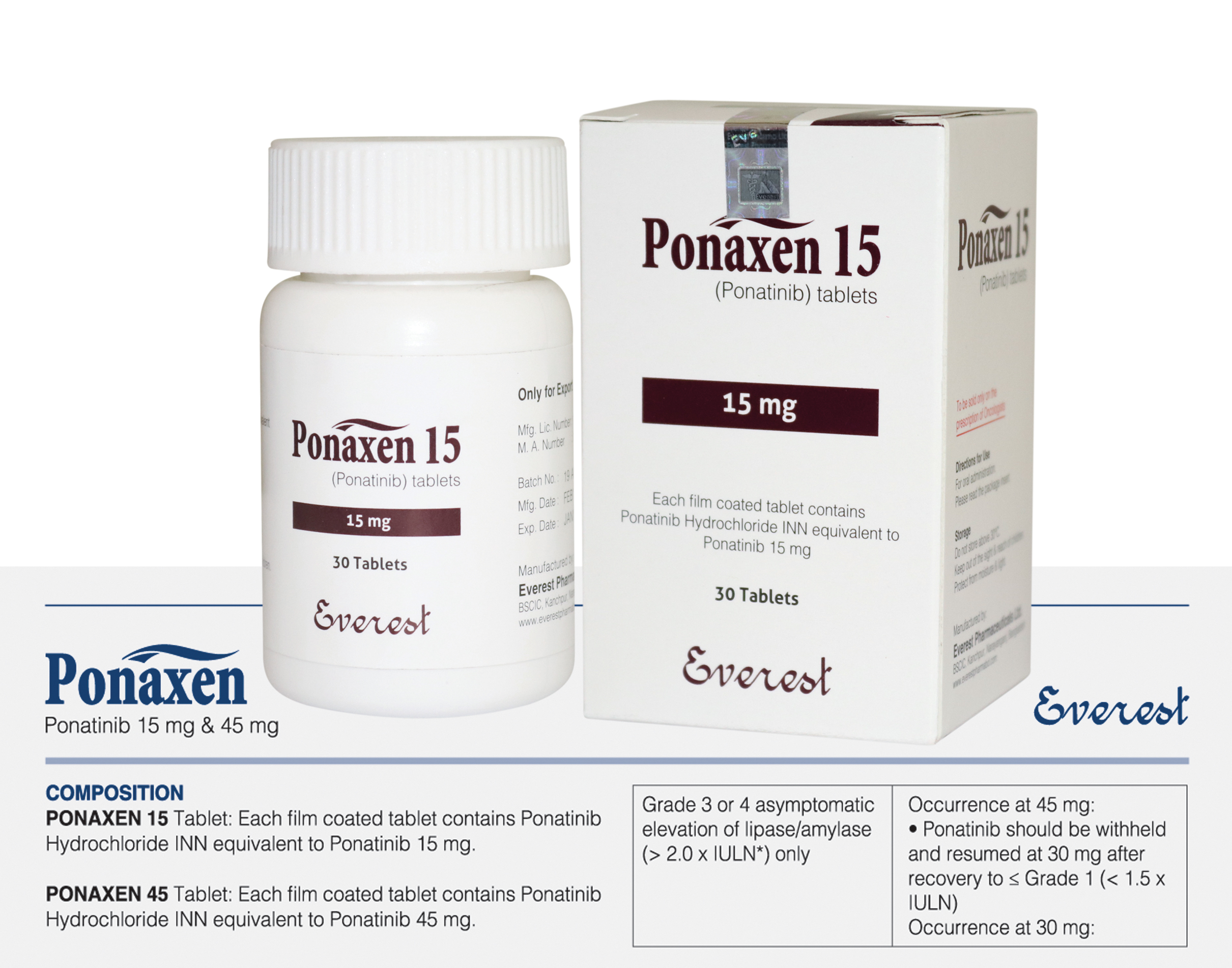
multi-target kinase inhibitor
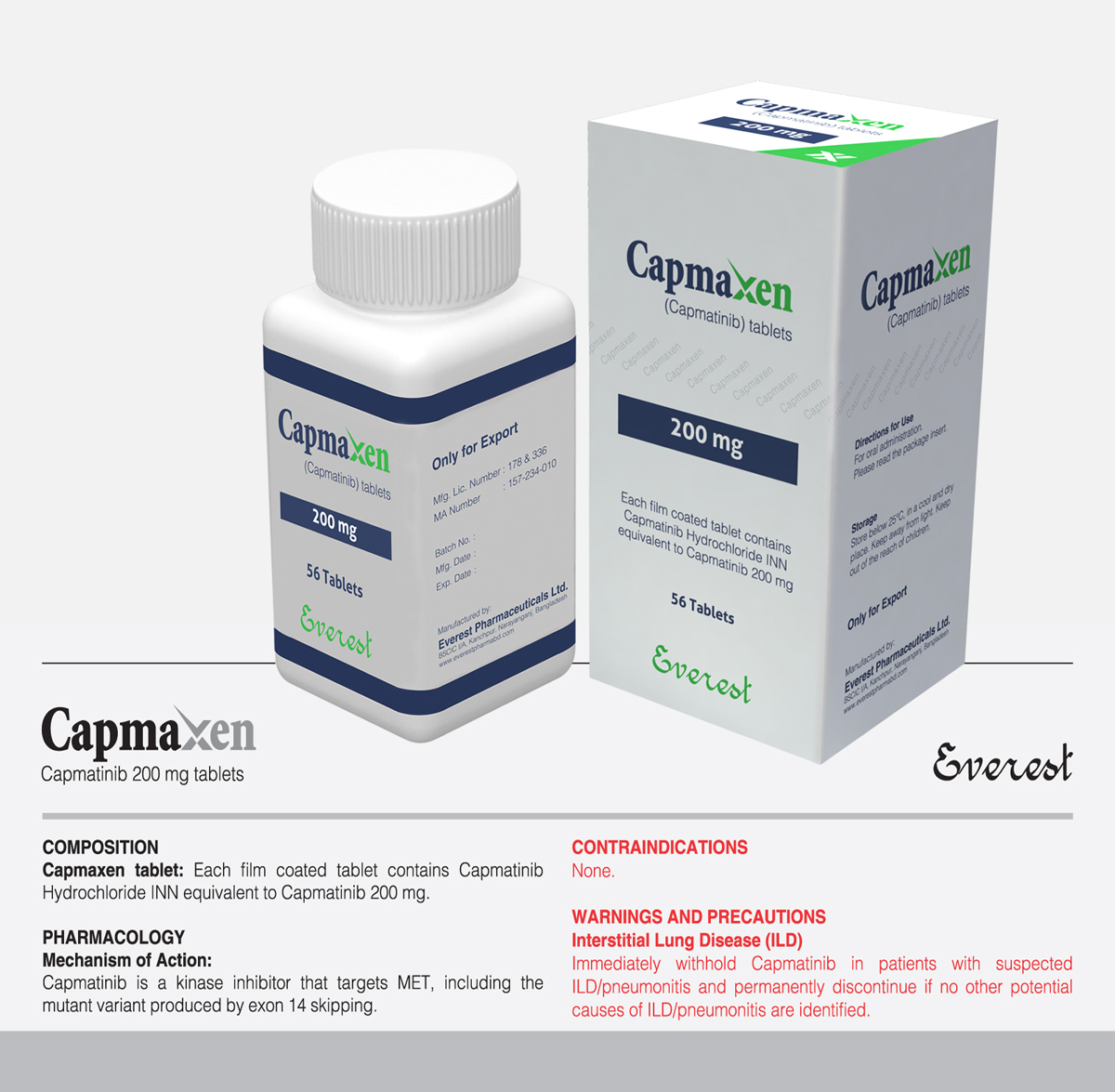
Tyrosin Kinase Inhibitor (TKI)
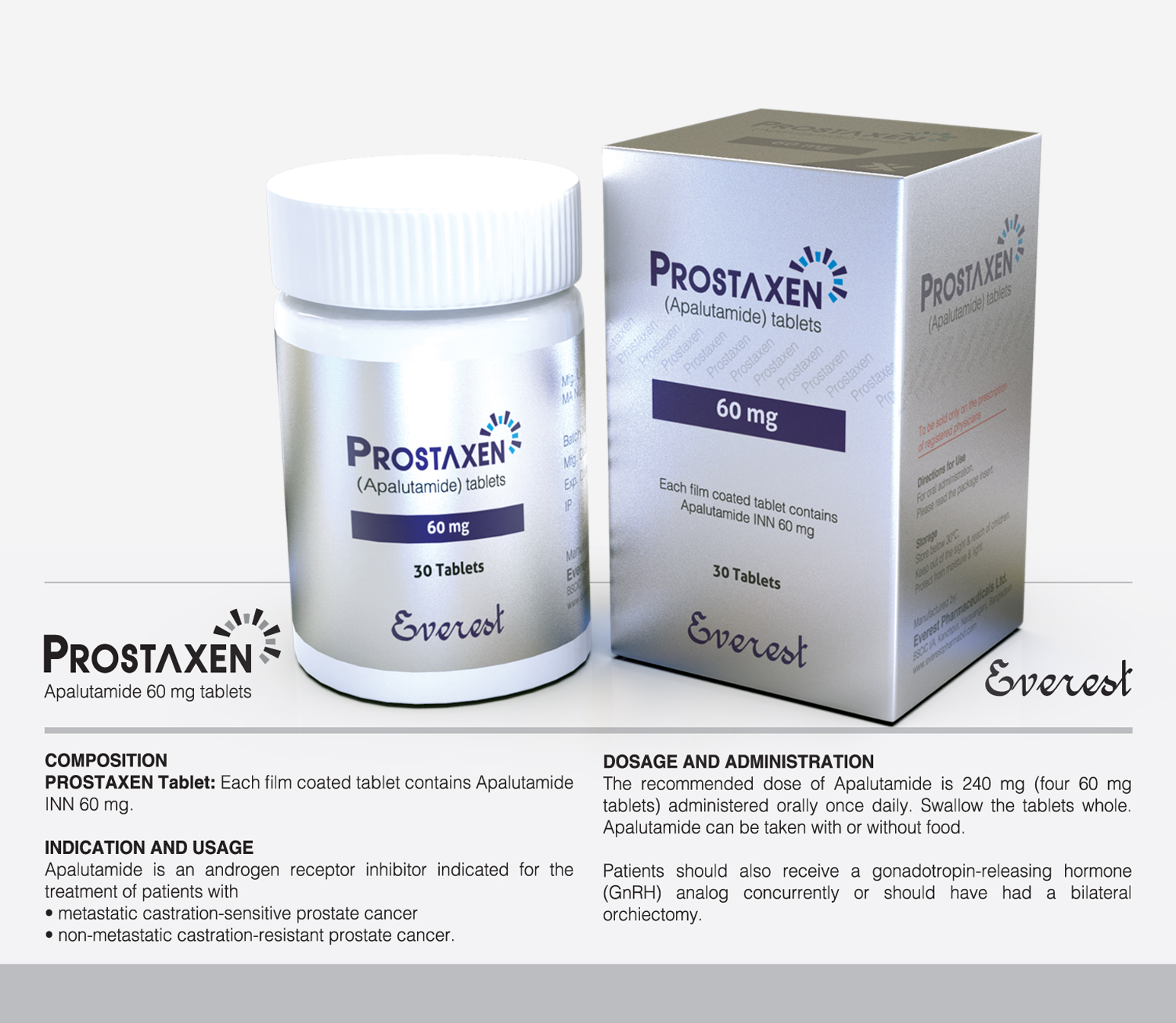
Androgen receptor inhibitors

B-cell lymphoma-2 (BCL-2) inhibitors

Mezoxia
Tyrosine kinase inhibitor