INDICATION AND USAGE :
Sotorasib is indicated for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test, who have received at least one prior systemic therapy.
DOSAGE AND ADMINISTRATION :The recommended dosage of Sotorasib is 960 mg (eight 120 mg tablets) orally once daily until disease progression or unacceptable toxicity. Take Sotorasib at the same time each day with or without food. Swallow tablets whole. Do not chew, crush or split tablets. If a dose of Sotorasib is missed by more than 6 hours, take the next dose as prescribed the next day. Do not take 2 doses at the same time to make up for the missed dose. If vomiting occurs after taking Sotorasib, do not take an additional dose. Take the next dose as prescribed the next day.
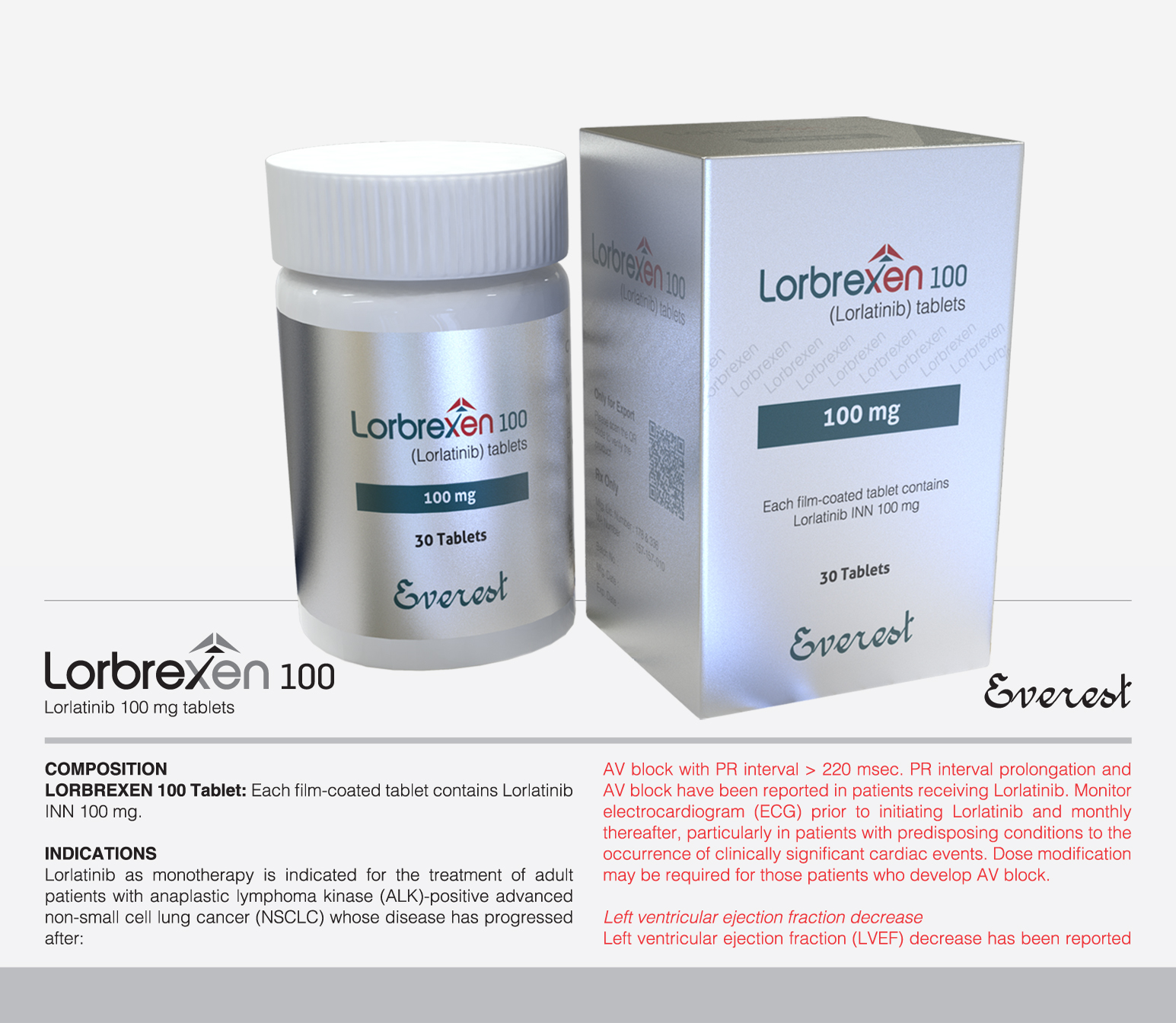
Tyrosine kinase inhibitor

Progestin class

Tyrosine kinase inhibitor
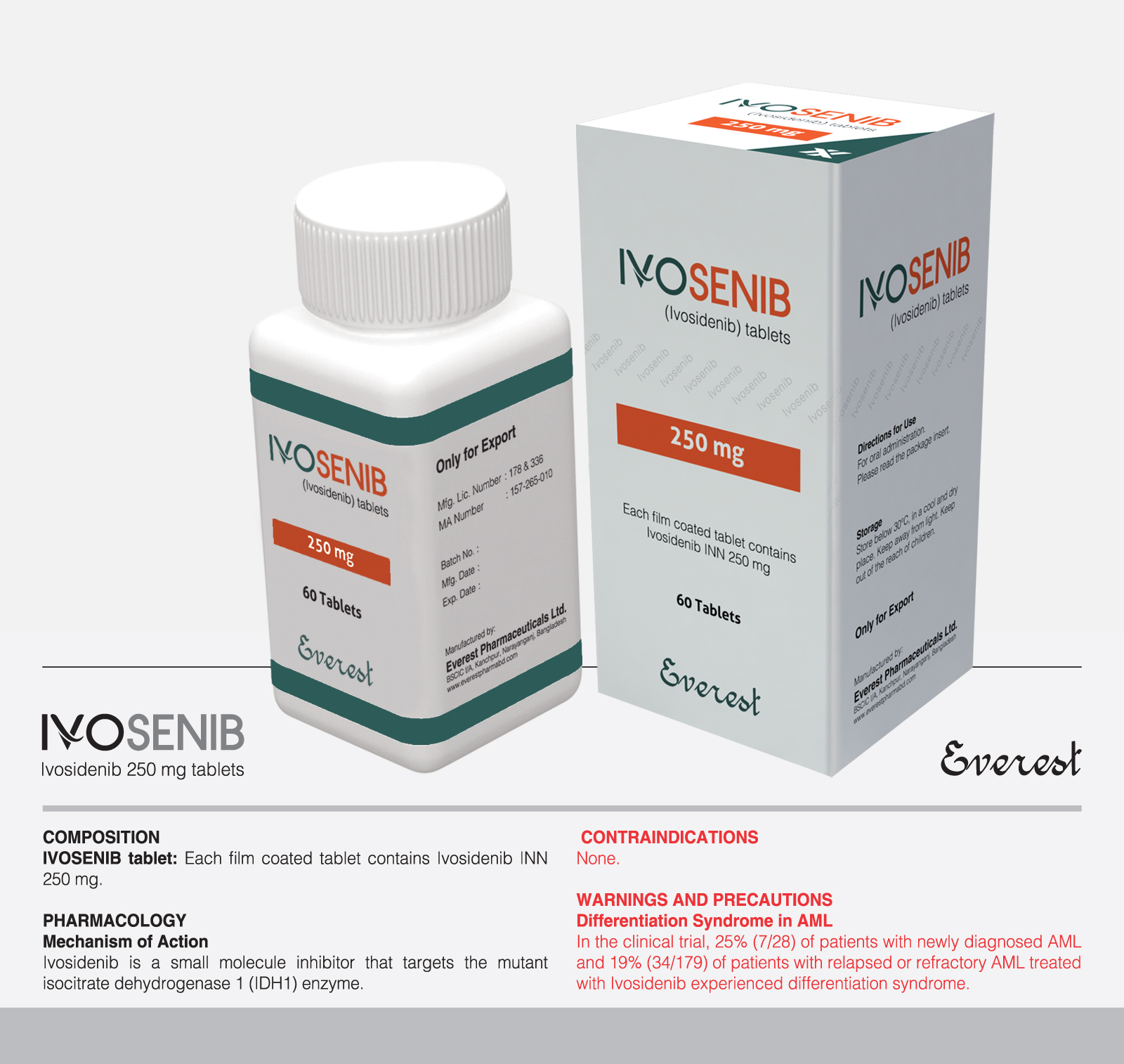
IDH1 inhibitor
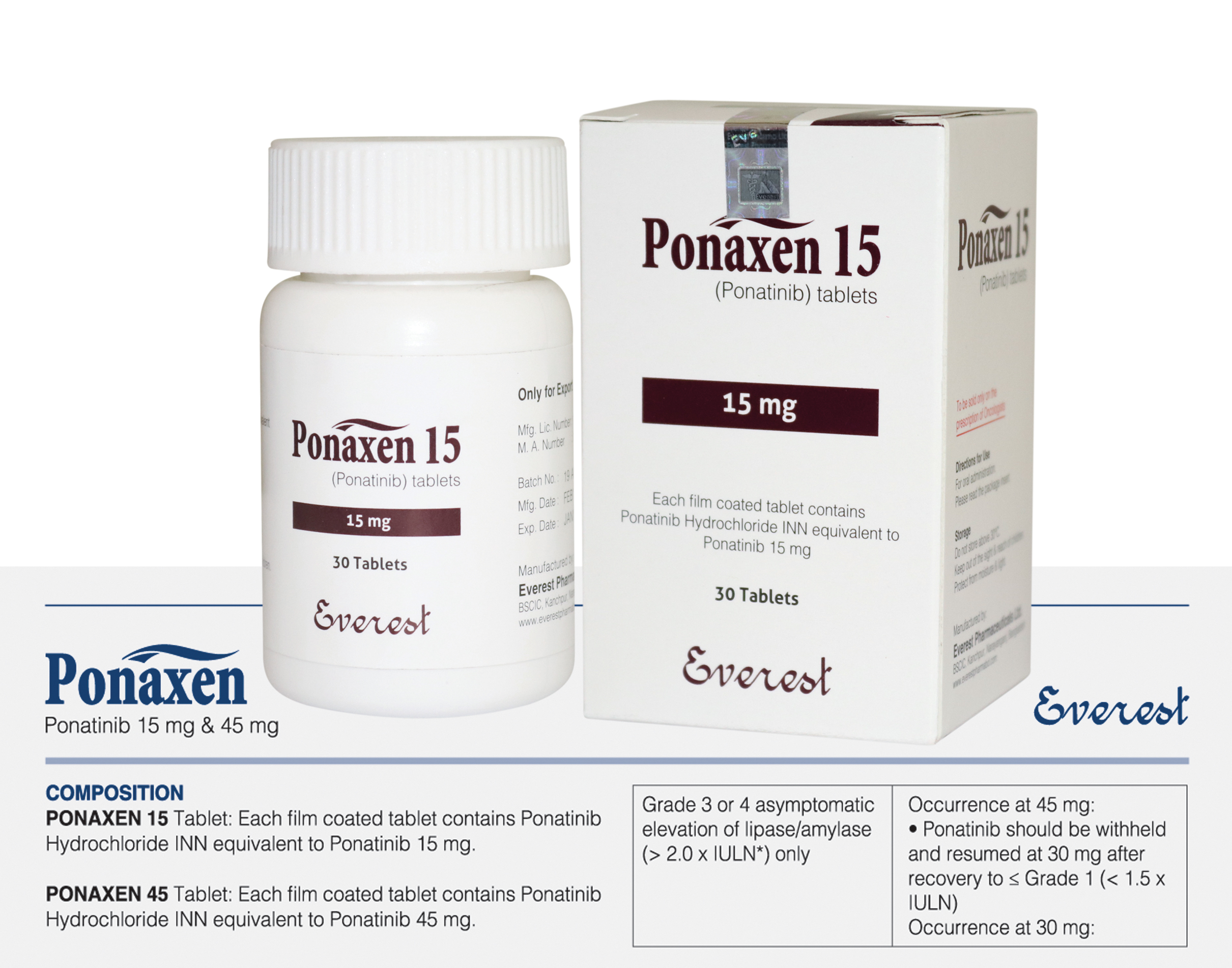
multi-target kinase inhibitor
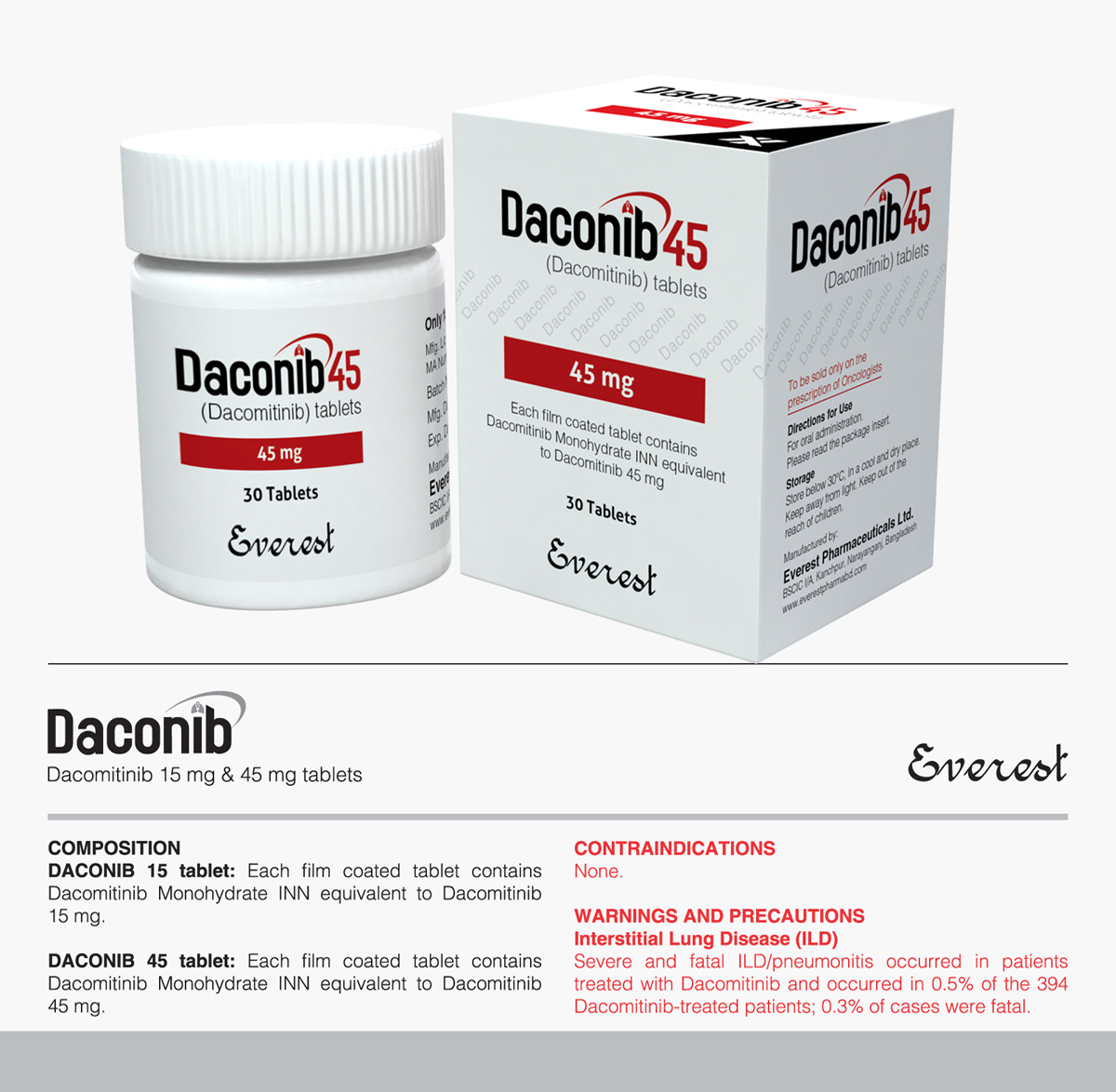
Tyrosin Kinase Inhibitor (TKI)
Tyrosine kinase inhibitor

polymerase (PARP) inhibitor

polymerase (PARP) inhibitors

KRAS inhibitors