INDICATIONS
Dacomitinib is indicated for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutations as detected by an FDA-approved test.
DOSAGE AND ADMINISTRATION
Patient Selection
Select patients for the first-line treatment of metastatic NSCLC with Dacomitinib based on the presence of an EGFR exon 19 deletion or exon 21 L858R substitution mutation in tumor specimens.
Recommended Dosage
The recommended dosage of Dacomitinib is 45 mg taken orally once daily, until disease progression or unacceptable toxicity occurs. Dacomitinib can be taken with or without food.
Take Dacomitinib the same time each day. If the patient vomits or misses a dose, do not take an additional dose or make up a missed dose but continue with the next scheduled dose.
Dosage Modifications for Adverse Reactions
Reduce the dose of Dacomitinib for adverse reactions as described in Table.
Table: Dacomitinib Recommended Dose Reductions for Adverse Reactions
| Dose Level | Dose (Once Daily) |
| First dose reduction | 30 mg |
| Second dose reduction | 15 mg |
Dosage Modifications for Acid-Reducing Agents
Avoid the concomitant use of proton pump inhibitors (PPIs) while taking Dacomitinib. As an alternative to PPIs, use locally-acting antacids or if using an histamine 2 (H2)-receptor antagonist, administer Dacomitinib at least 6 hours before or 10 hours after taking an H2-receptor antagonist.
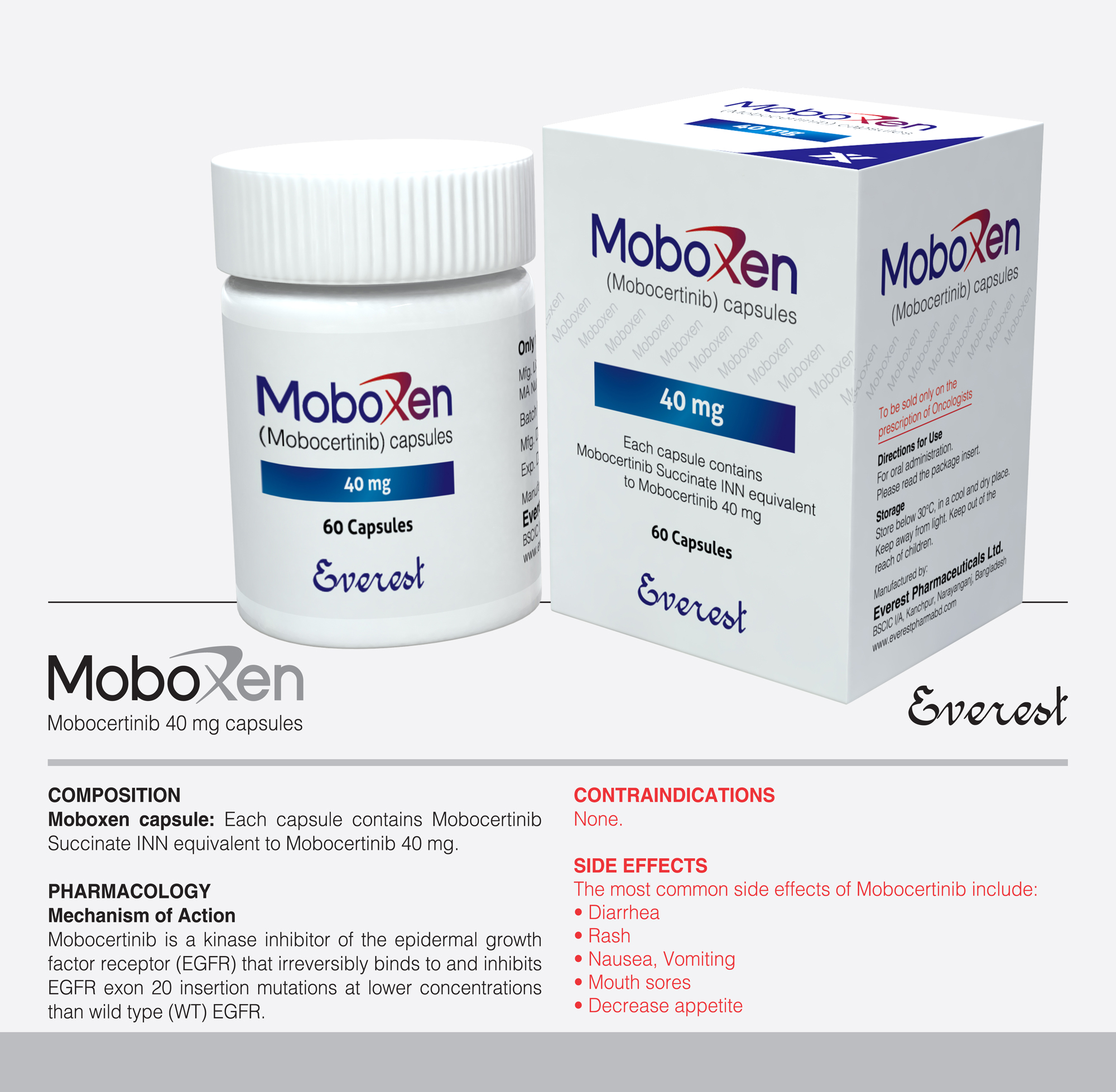
Tyrosine kinase inhibitor
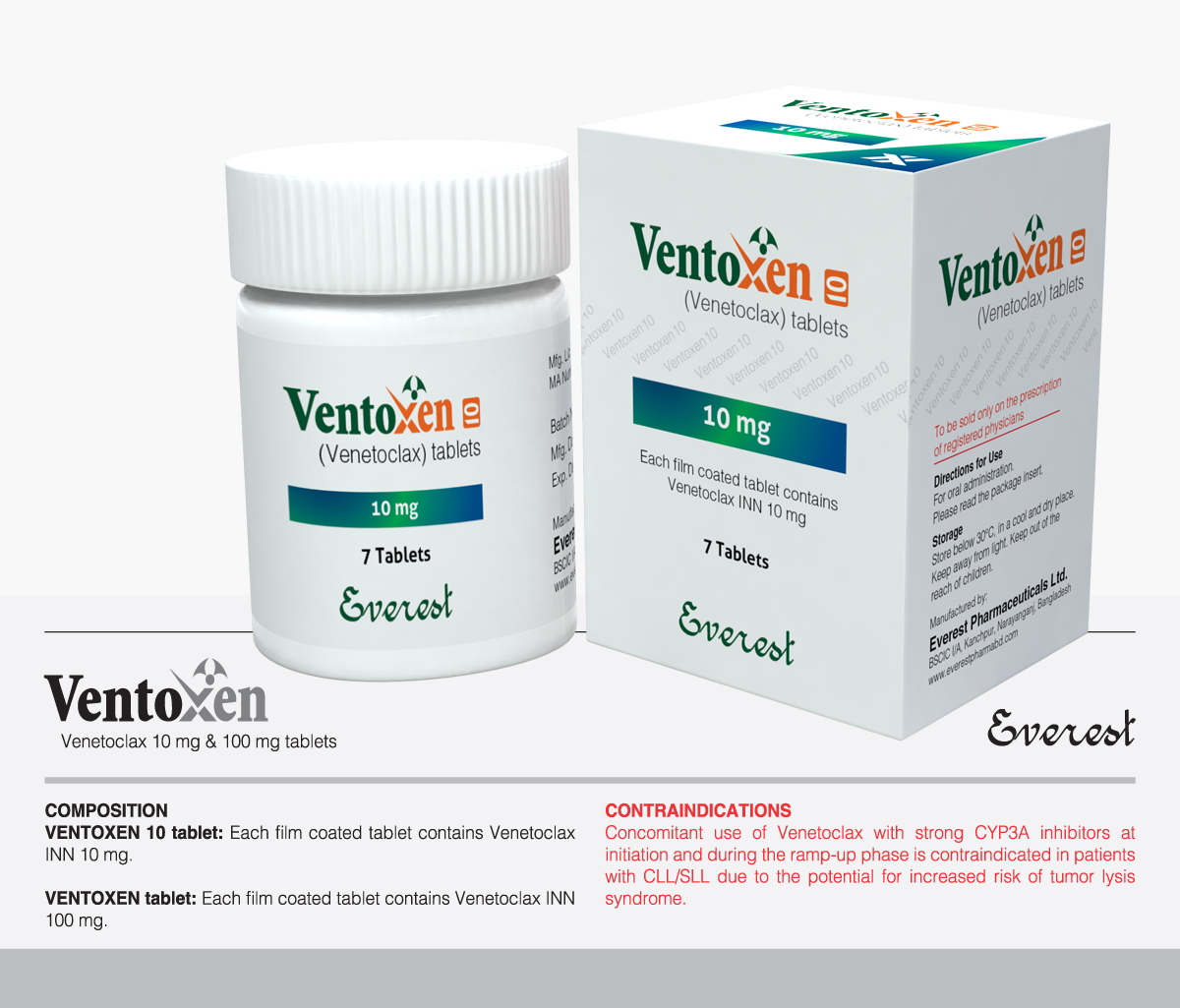
B-cell lymphoma-2 (BCL-2) inhibitors
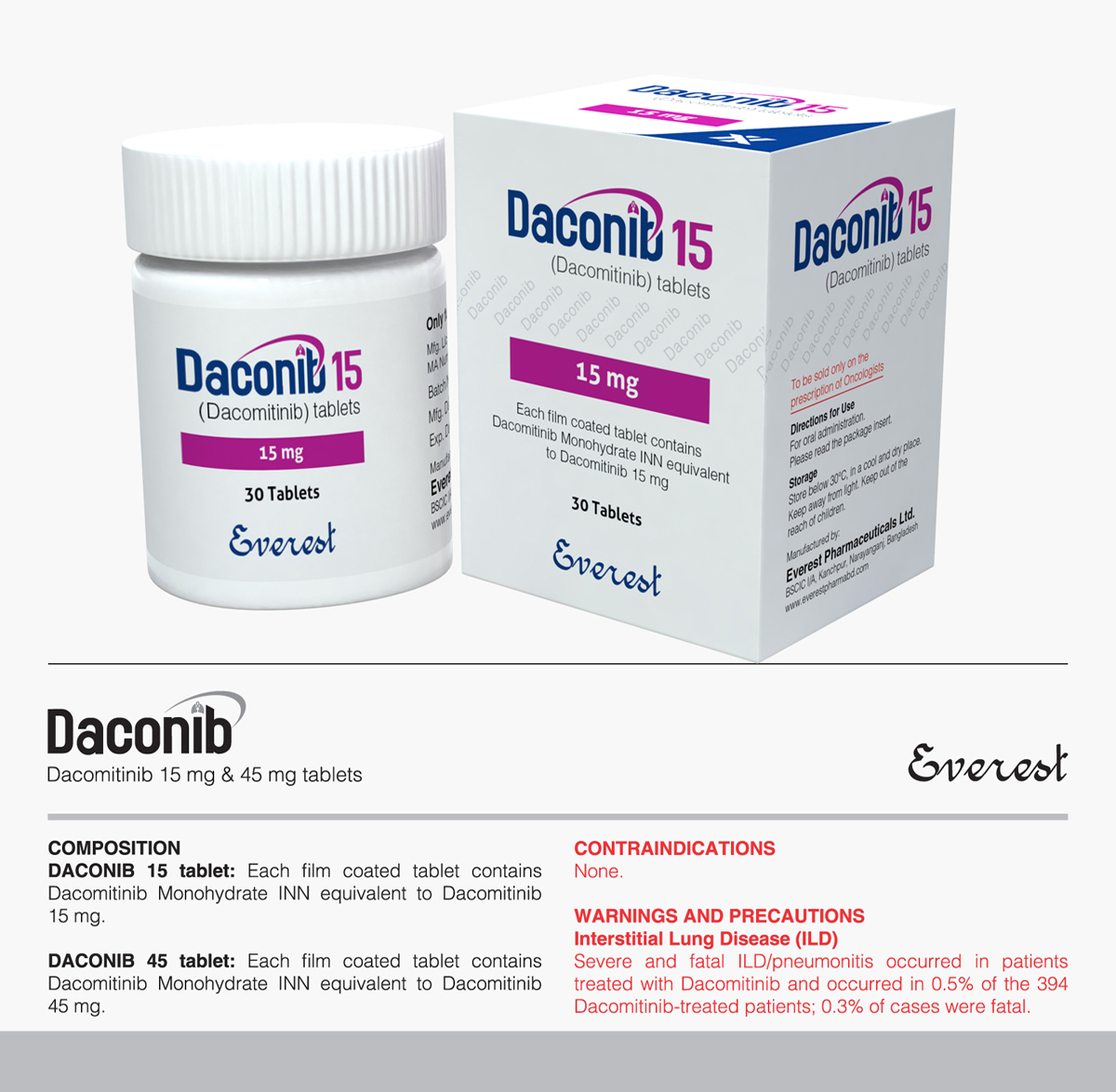
Tyrosin Kinase Inhibitor (TKI)
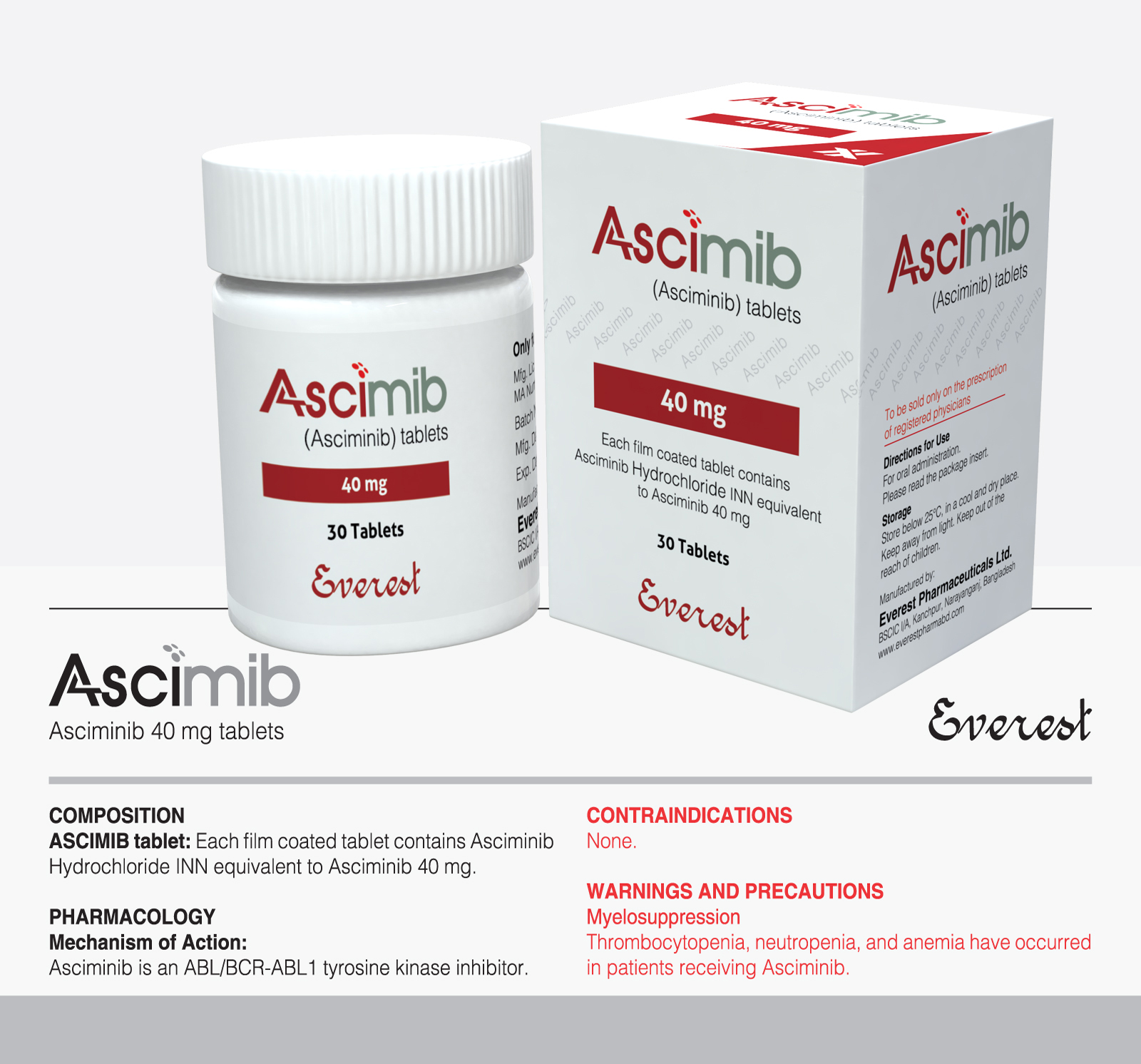
Tyrosine kinase inhibitor

Tyrosine kinase inhibitor

Renal Cell Carcinoma

Progestin class

kinase inhibitors
Tyrosine kinase inhibitor

Tyrosine kinase inhibitor