INDICATION AND USAGE
Metastatic RET Fusion-Positive Non-Small Cell Lung Cancer treatment of adul Selpercatinib is indicated for the patients with metastatic RET fusion-positive non-small cell lung cancer (NSCLC). This indication is approved under accelerated approval based on overall response rate and duration of response.. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. RET-Mutant Medullary Thyroid Cancer Selpercatinib is indicated for the treatment of adult and pediatric patients 12 years of age and older with advanced or metastatic RET-rutant medullary thyroid cancer (MTC) who require systemic therapy. This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. RET Fusion-Positive Thyroid Cancer Selpercatinib is indicated for the treatment of adult and pediatric patients 12 years of age and older with advanced or metastatic RET fusion-positive thyroid cancer who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate). This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials).
DOSAGE AND ADMINISTRATION: Patient Selection Select patients for treatment with Selbercatinib based on the presence of a RET gene fision (NSCLC or thyroid cancer) or specific RET gene mutation (MTC) in tumor specimens or plasma. An FDA-approved test for the detection of RET gene fusions and RET gene mutations is not currently available. Important Administration Instructions SELPERCATINIB may be taken with or without food unless co-administrated with a proton pump inhibitor (PPI).

Tyrosine kinase inhibitor

Renal Cell Carcinoma
Tyrosine kinase inhibitor

Tyrosine kinase inhibitor
Tyrosine kinase inhibitor

KRAS inhibitors
Tyrosine kinase inhibitor
Androgen receptor inhibitors
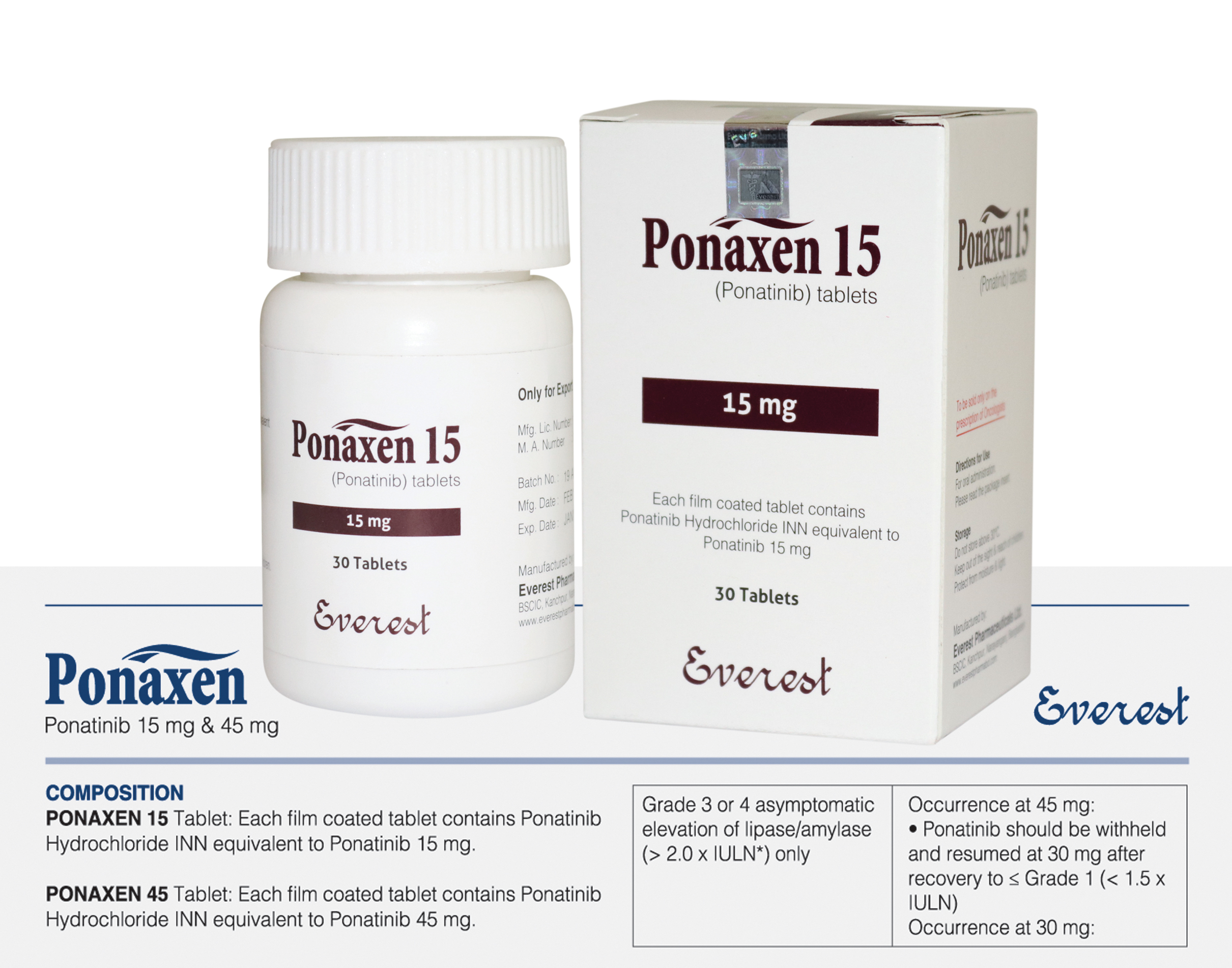
multi-target kinase inhibitor
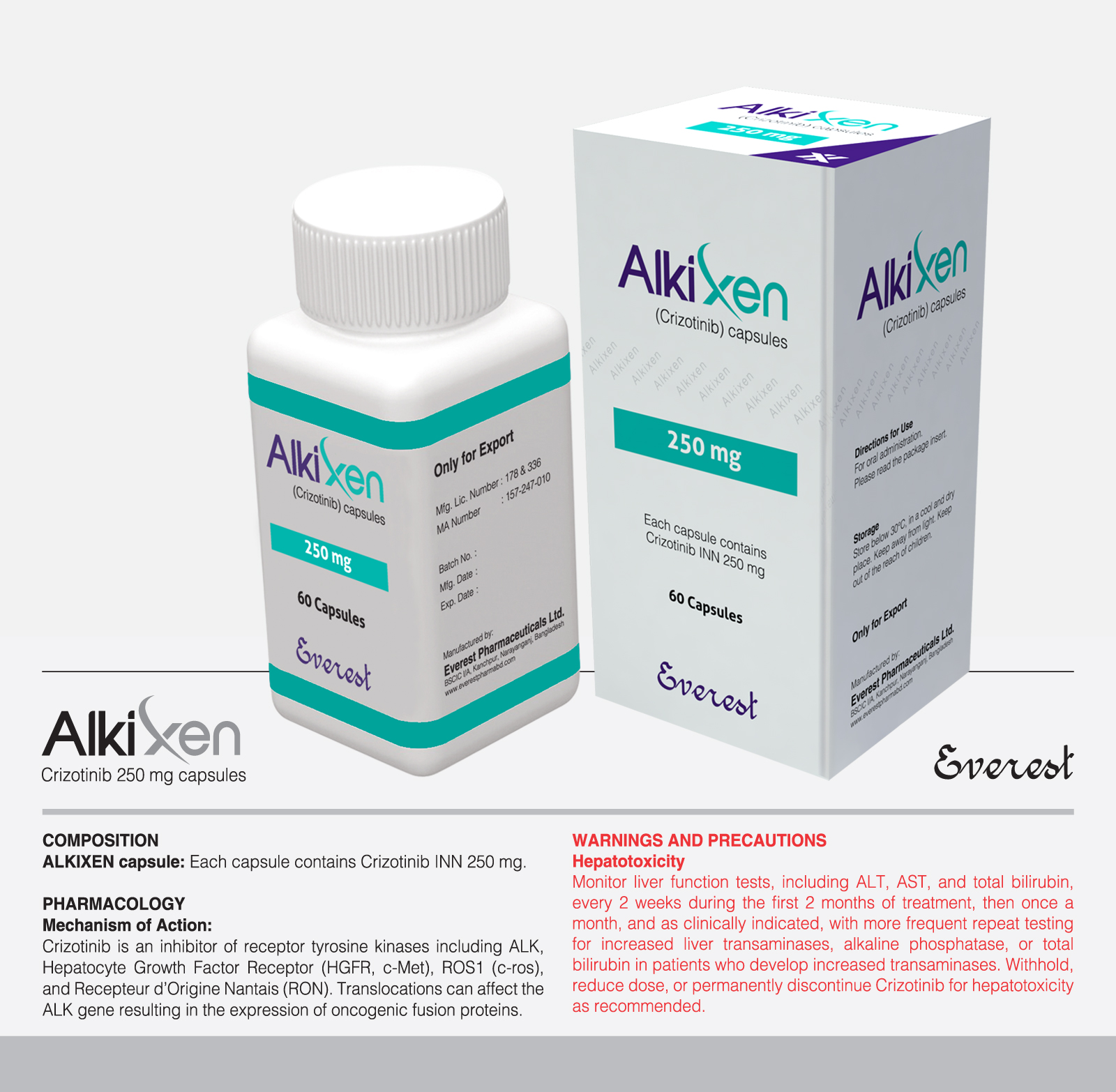
Tyrosine kinase inhibitor