INDICATIONS AND USAGE
Newly-Diagnosed Acute Myeloid Leukemia Ivosidenib is indicated for the treatment of newly-diagnosed acute myeloid leukemia (AML) with a susceptible isocitrate dehydroge- nase-1 (IDH1) mutation as detected by an FDA-approved test in adult patients who are ≥75 years old or who have comorbidities that preclude use of intensive induction chemotherapy.
Relapsed or Refractory Acute Myeloid Leukemia Ivosidenib is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.
Locally Advanced or Metastatic Cholangiocarcinoma Ivosidenib is indicated for the treatment of adult patients with previously treated, locally advanced or metastatic cholangiocarcinoma with an isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.
DOSAGE AND ADMINISTRATION Patient Selection
Acute Myeloid Leukemia
Select patients for the treatment of AML with Ivosidenib based on the presence of IDH1 mutations in the blood or bone marrow. Patients with AML without IDH1 mutations at diagnosis should be retested at relapse because a mutation in IDH1 may emerge during treatment and at relapse.
Locally Advanced or Metastatic Cholangiocarcinoma Select patients for the treatment of locally advanced or metastatic cholangiocarcinoma with Ivosidenib based on the presence of IDH1 mutations.
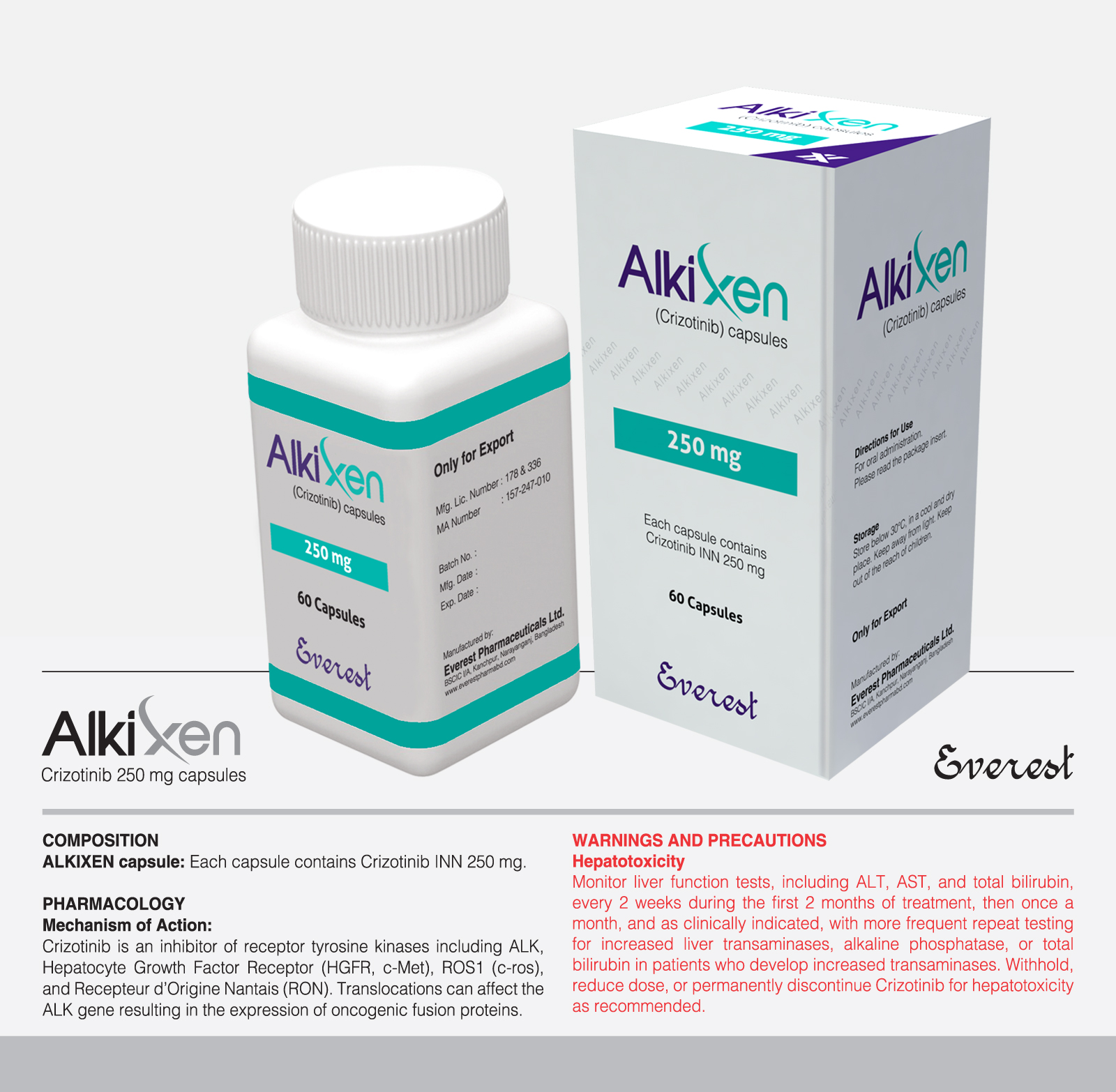
Tyrosine kinase inhibitor
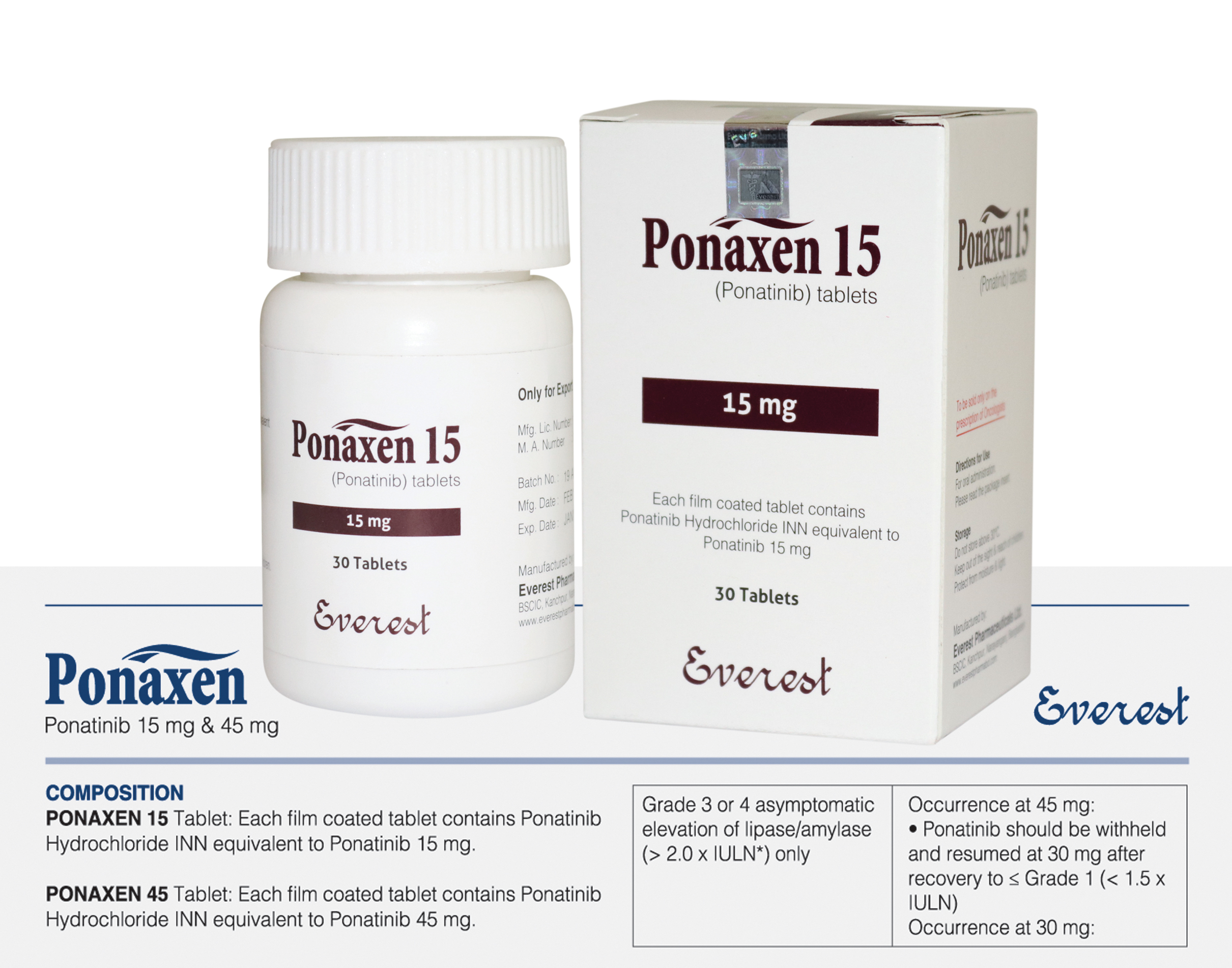
multi-target kinase inhibitor
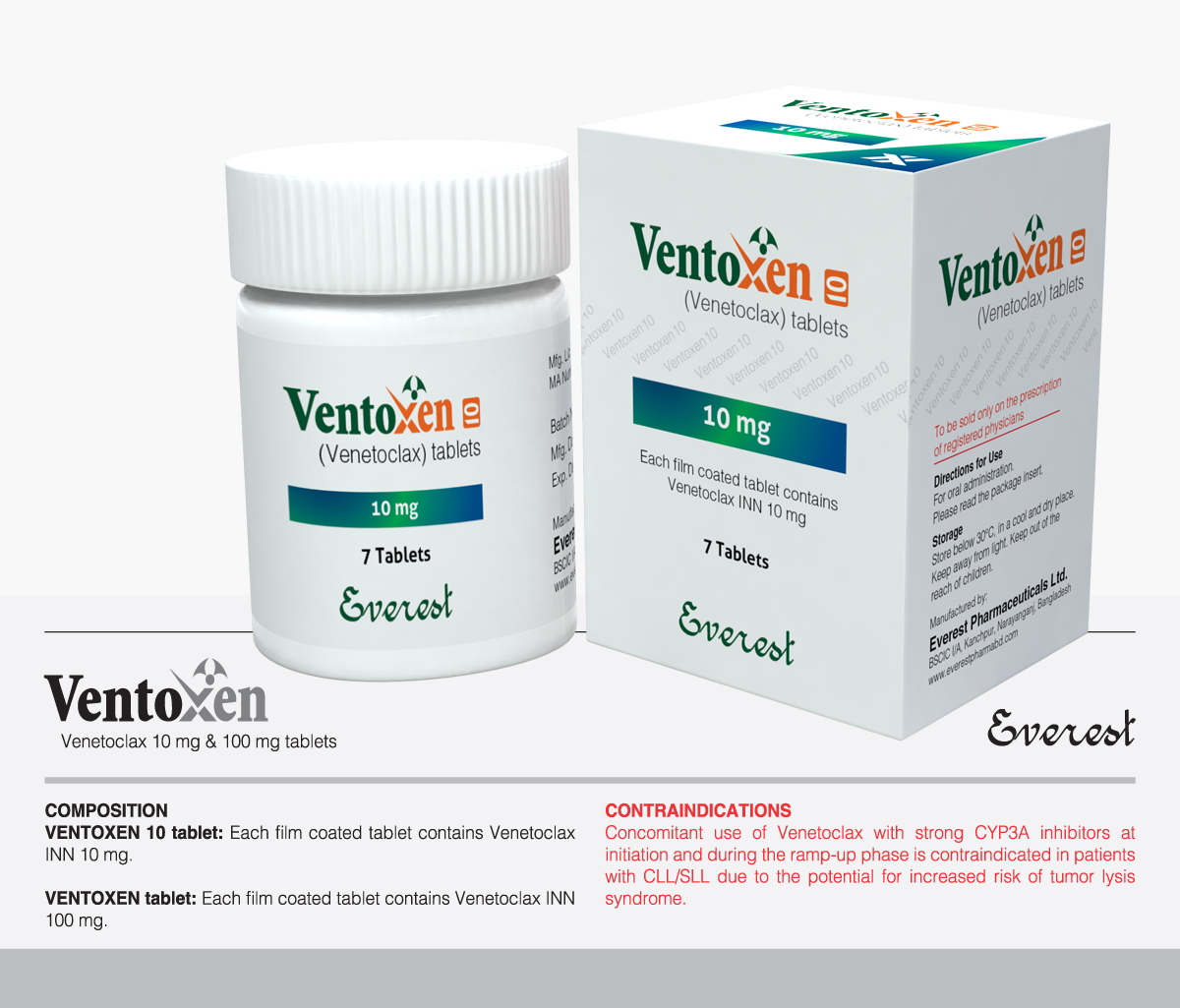
B-cell lymphoma-2 (BCL-2) inhibitors

Renal Cell Carcinoma

Tyrosine kinase inhibitor
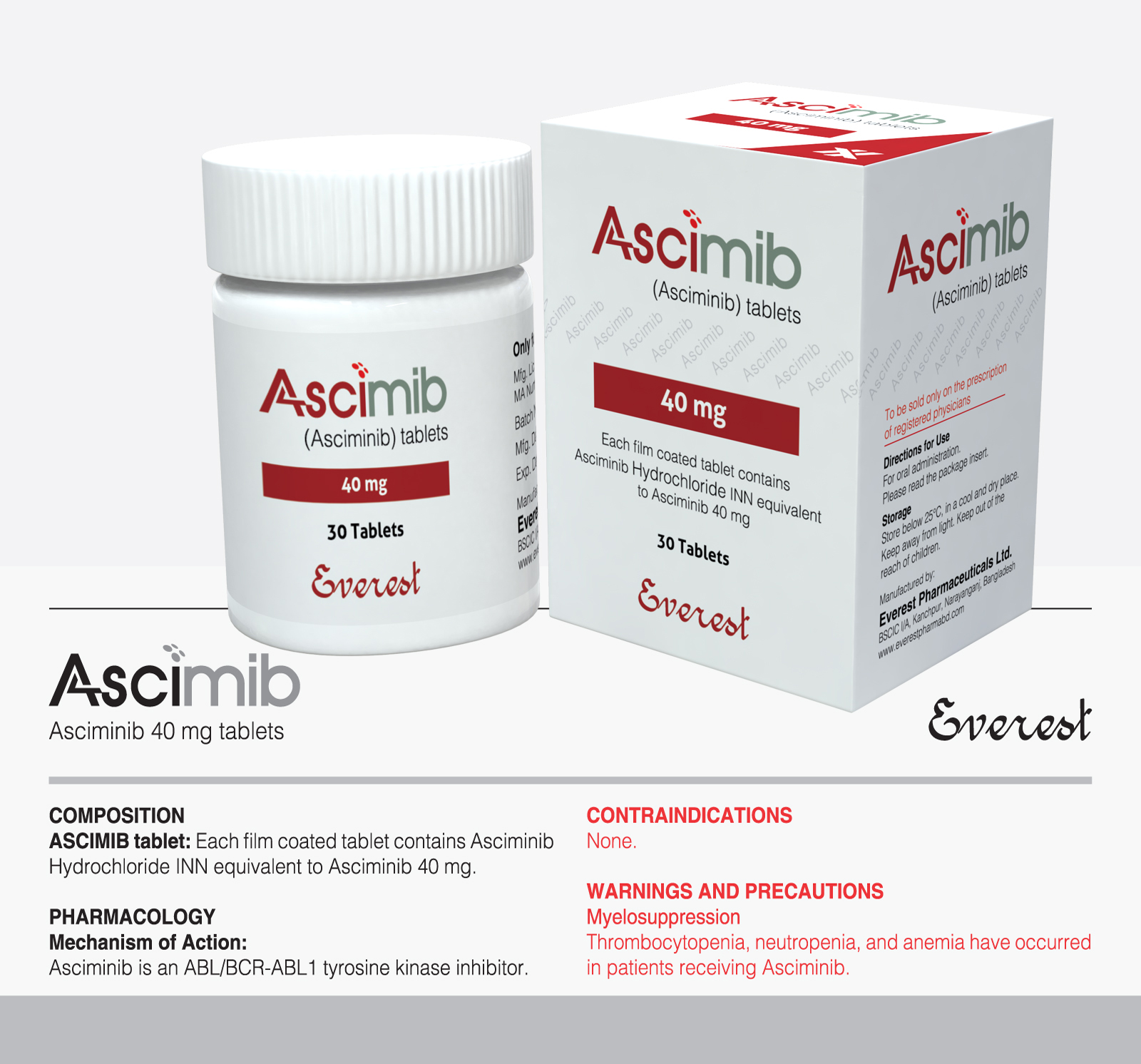
Tyrosine kinase inhibitor

Progestin class
Tyrosin Kinase Inhibitor (TKI)

Tyrosine kinase inhibitor
IDH1 inhibitor