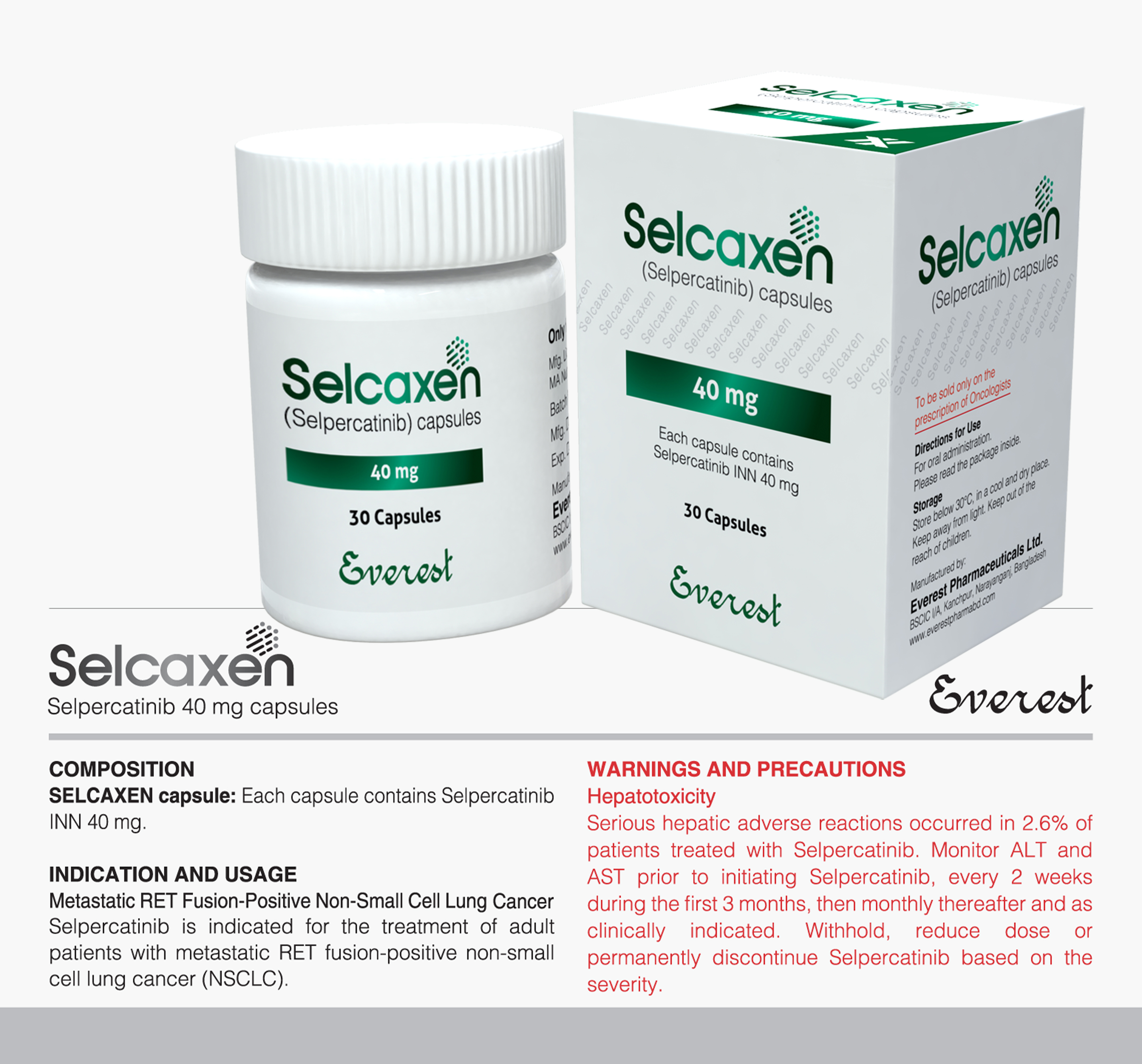
INDICATIONS AND USAGE :
Mantle Cell Lymphoma Ibrutinib (Ibruxen) is indicated for the treatment of patients with Mantle Cell Lymphoma (MCL) who have received at least one prior therapy. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Ibrutinib (Ibruxen) is indicated for the treatment of adult patients with chronic lymphocytic leukemia (CLL)/ small lymphocytic lymphoma (SLL), Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma with 17p deletion Ibrutinib (Ibruxen) is indicated for the treatment of patients with chronic lymphocytic leukemia (CLL)/ small lymphocytic lympho- ma (SLL) with 17p deletion. Waldenstrom Macroglobulinemia (WM) Ibrutinib (Ibruxen) is indicated for the treatment of patients with Waldenstrom Macroglobulinemia (WM). Marginal Zone Lymphoma Ibrutinib (Ibruxen) is indicated for the treatment of patients with marginal zone lymphoma (MZL) who require systemic therapy and have received at least one prior anti-CD20-based therapy. Chronic Graft versus Host Disease Ibrutinib (Ibruxen) is indicated for the treatment of adult patients with chronic graft-versus-host disease (cGVHD) after failure of one or more lines of systemic therapy
DOSAGE AND ADMINISTRATION:Dosing Guidelines Administer Ibrutinib (Ibruxen) orally once daily at approximately the same time each day. Swallow the capsules whole with water. Do not open, break, or chew the capsules,
Recommended Dosage:Mantle Cell Lymphoma and Marginal Zone Lymphoma The recommended dose of Ibrutinib (Ibruxen) for MCL and MZL is 560 mg (four 140 mg capsules) orally once daily until disease progression or unacceptable toxicity. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma and Waldenstrom Macroglobulinemia (WM) The recommended dose of Ibrutinib (lbruxen) for CLL/SLL and WM is 420 mg (three 140 mg capsules) orally once daily until disease progression or unacceptable toxicity. The recommend- ed dose of Ibrutinib for CLL/SLL when used in combination with bendamustine and rituximab (administered every 28 days for up to 6 cycles) is 420 mg (three 140 mg capsules) orally once daily until disease progression or unacceptable toxicity. Chronic Graft versus Host Disease The recommended dose of Ibrutinib (lbruxen) for cGVHD is 420 mg (three 140 mg capsules) orally once daily until cGVHD progression, recurrence of an underlying malignancy, or unacceptable toxicity. When a patient no longer requires therapy for the treatment of cGVHD, Ibrutinib should be discon- tinued considering the medical assessment of the individual patient.
Dose Modifications for Adverse Reactions Interrupt Ibrutinib therapy for any Grade 3 or greater non-hema- tological toxicities, Grade 3 or greater neutropenia with infection or fever, or Grade 4 hematological toxicities. Once the symptoms of the toxicity have resolved to Grade 1 or baseline (recovery). Ibrutinib therapy may be reinitiated at the starting dose. If the toxicity reoccurs, reduce dose by one capsule (140 mg per day). A second reduction of dose by 140 mg may be considered as needed. If these toxicities persist or recur following two dose reductions, discontinue Ibrutinib.
Dose Modifications for Use with CYP3A Inhibitors Avoid co-administration with strong or moderate CYP3A inhibitors and consider alternative agents with less CYP3A inhibition. Concomitant use of strong CYP3A inhibitors which would be taken chronically (e.g., ritonavir, indinavir, nelfinavir, saquinavir, boceprevir, telaprevir, nefazodone) is not recommended. For short-term use (treatment for 7 days or less) of strong CYP3A inhibitors (e.g., antifungals and antibiotics) consider interrupting Ibrutinib therapy until the CYP3A inhibitor is no longer needed. Reduce Ibrutinib dose to 140 mg if a moderate CYP3A inhibitor must be used (e.g., fluconazole, darunavir, erythromycin, diltiazem, atazanavir, aprepitant. amprenavir, fosamprenavir, crizotinib, imatinib, verapamil, and ciprofloxacin). Patients taking concomitant strong or moderate CYP3A inhibitors should be monitored more closely for signs of Ibrutinib toxicity. Dose Modifications for Use in Hepatic Impairment For patients with mild liver impairment (Child-Pugh class A), the recommended dose is 140 mg daily (one capsule). Avoid the use of Ibrutinib in patients with moderate or severe hepatic impairment (Child-Pugh classes Band C). Missed Dose If a dose of Ibrutinib is not taken at the scheduled time, it can be taken as soon as possible on the same day with a return to the normal schedule the following day. Extra capsules of Ibrutinib should not be taken to make up for the missed dose.
Tyrosine kinase inhibitor

Tyrosine kinase inhibitor

Tyrosine kinase inhibitor

Tyrosine kinase inhibitor
Tyrosine kinase inhibitor

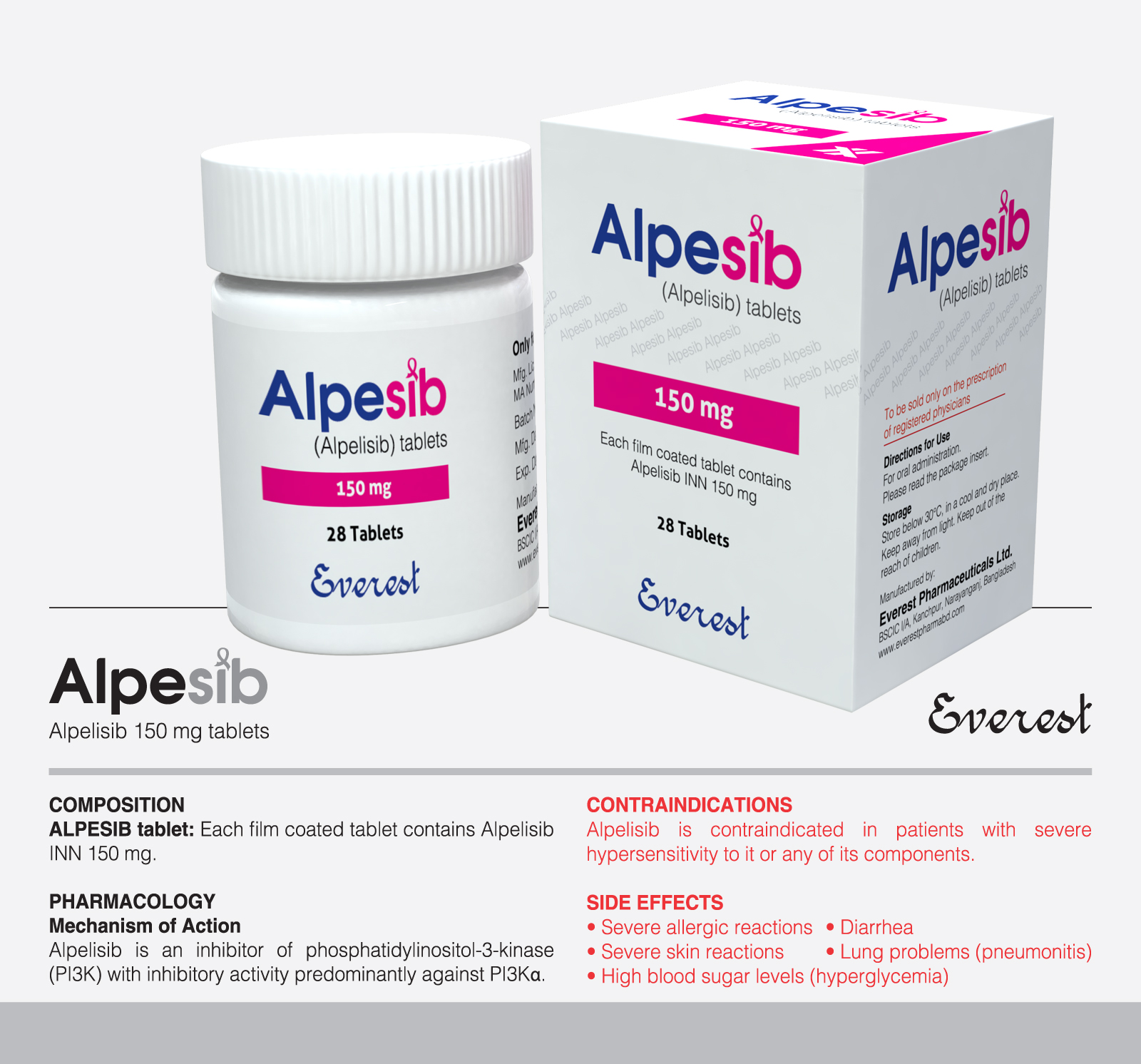
Mezoxia

B-cell lymphoma-2 (BCL-2) inhibitors

polymerase (PARP) inhibitor

polymerase (PARP) inhibitors

fluoropyrimidines