INDICATIONS AND USAGE:
Alpelisib is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CAmutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen.
DOSAGE AND ADMINISTRATION: The recommended dose of Alpelisib is 300 mg (two 150 mg film coated tablets) taken orally, once daily, with food. Continue treatment until disease progression or unaccept- able toxicity occurs. Patients should take their dose of Alpelisib at approximately the same time each day. Swallow Alpelisib tablets whole (tablets should not be chewed, crushed or split prior to swallowing). No tablet should be ingested if it is broken, cracked, or otherwise not intact. If a dose of Alpelisib is missed, it can be taken with food within 9 hours after the time it is usually taken. After more than 9 hours, skip the dose for that day. The next day, take Alpelisib at the usual time.
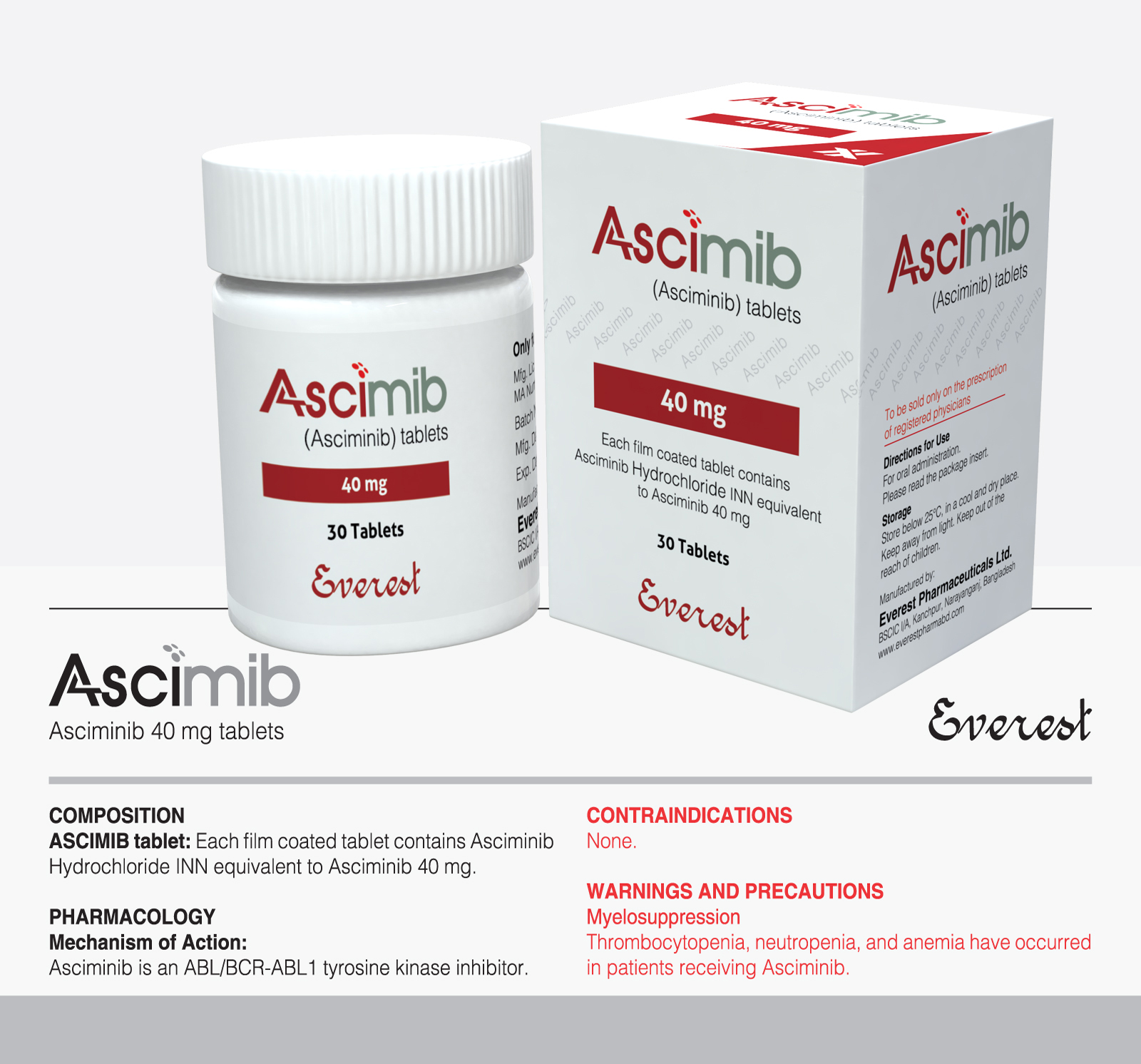
Tyrosine kinase inhibitor

Tyrosine kinase inhibitor

KRAS inhibitors
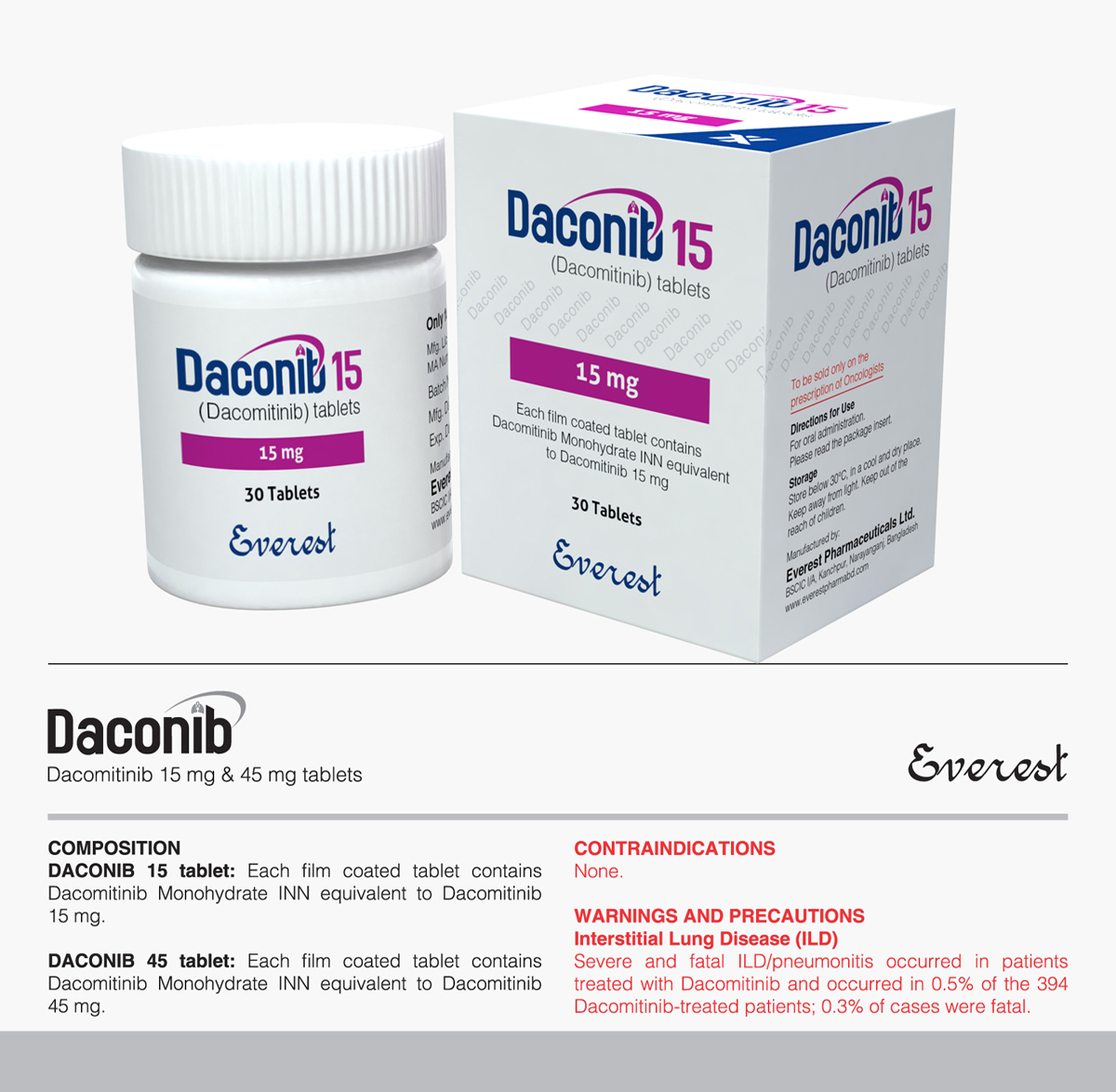
Tyrosin Kinase Inhibitor (TKI)
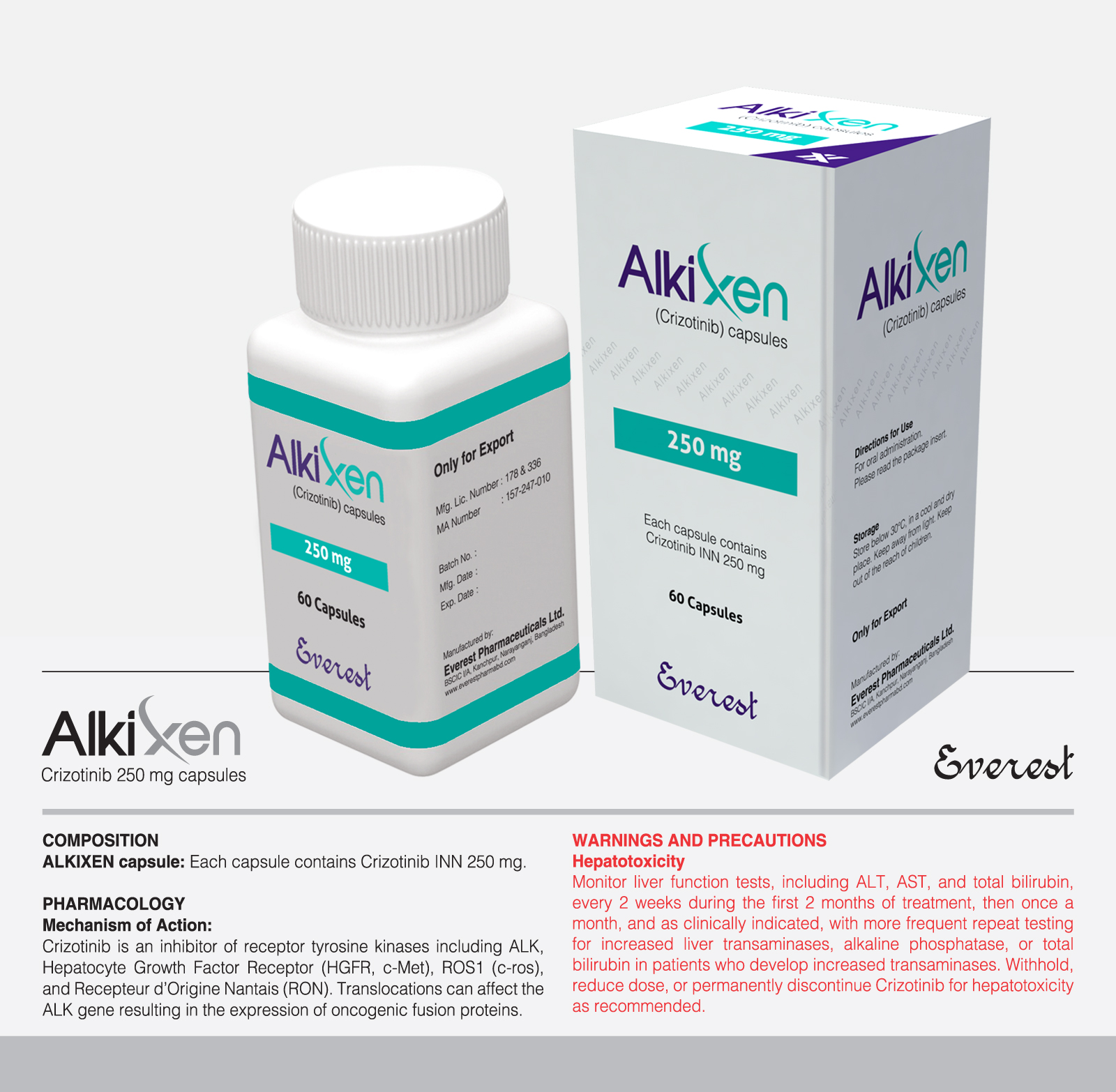
Tyrosine kinase inhibitor

polymerase (PARP) inhibitors

Tyrosine kinase inhibitor
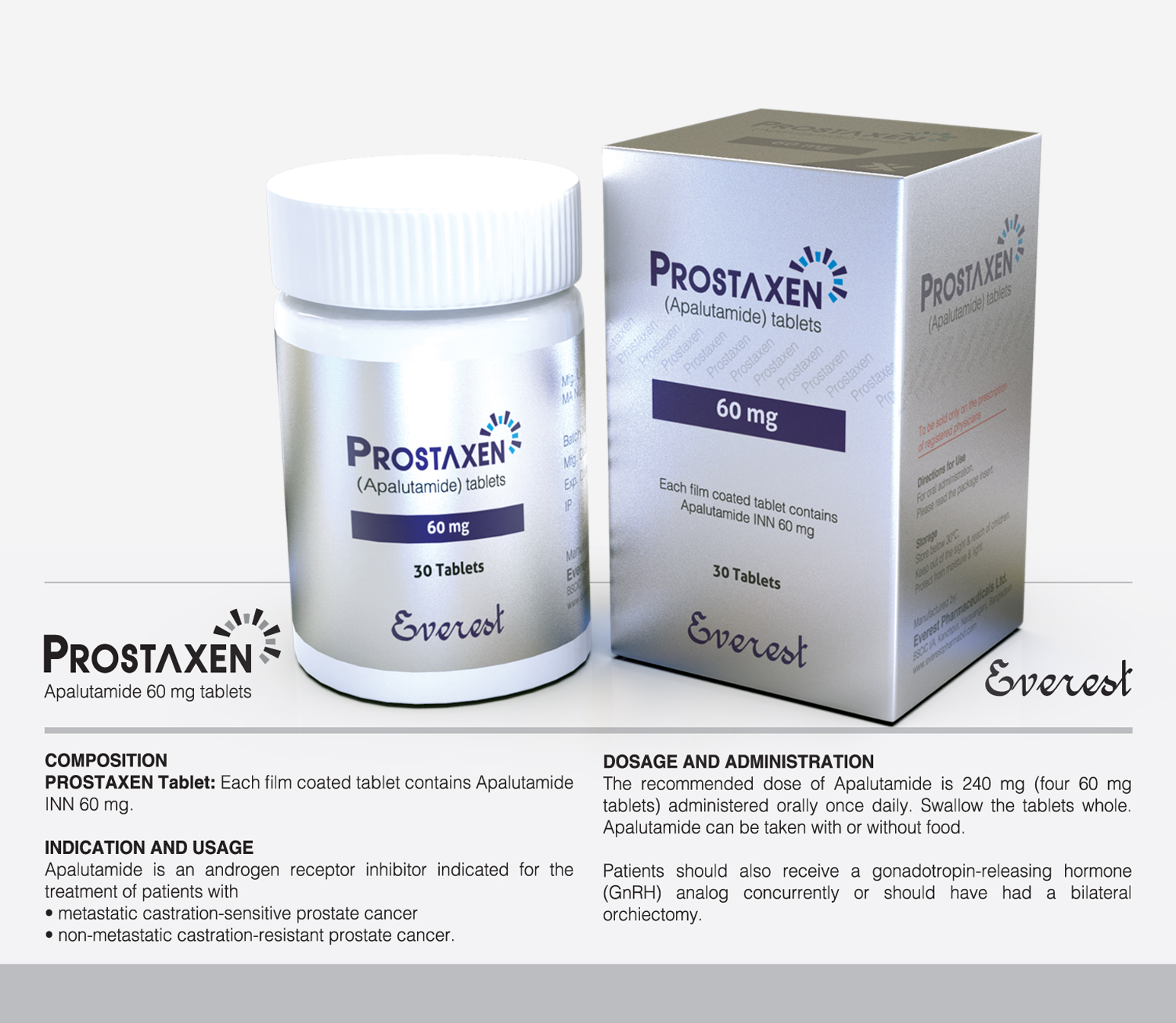
Androgen receptor inhibitors

B-cell lymphoma-2 (BCL-2) inhibitors

Mezoxia