INDICATIONS AND USAGE Metastatic Colorectal Cancer Trifluridine and Tipiracil is indicated for the treatment of adult patients with metastatic colorectal cancer previously treated with fluoropyrimidine, oxaliplatin-and irinotecan- based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy
Metastatic Gastric Cancer Trifluridine and Tipiracil is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate, HER2/neu-targeted therapy. DOSAGE AND ADMINISTRATION Recommended Dosage The recommended dosage of Trifluridine and Tipiracil is 35 mg/m² up to a maximum of 80 mg per dose (based on the Trifluridine component) orally twice daily with food on Days 1 through 5 and Days 8 through 12 of each 28-day cycle until disease progression or unacceptable toxicity. Round dose to the nearest 5 mg increment. Instruct patients to swallow the tablet whole. Instruct patients not to retake doses of Trifluridine and Tipiracil that are vomited or missed and to continue with the next scheduled dose. Trifluridine and Tipiracil is a cytotoxic drug. Follow applicable special handling and disposal procedures.
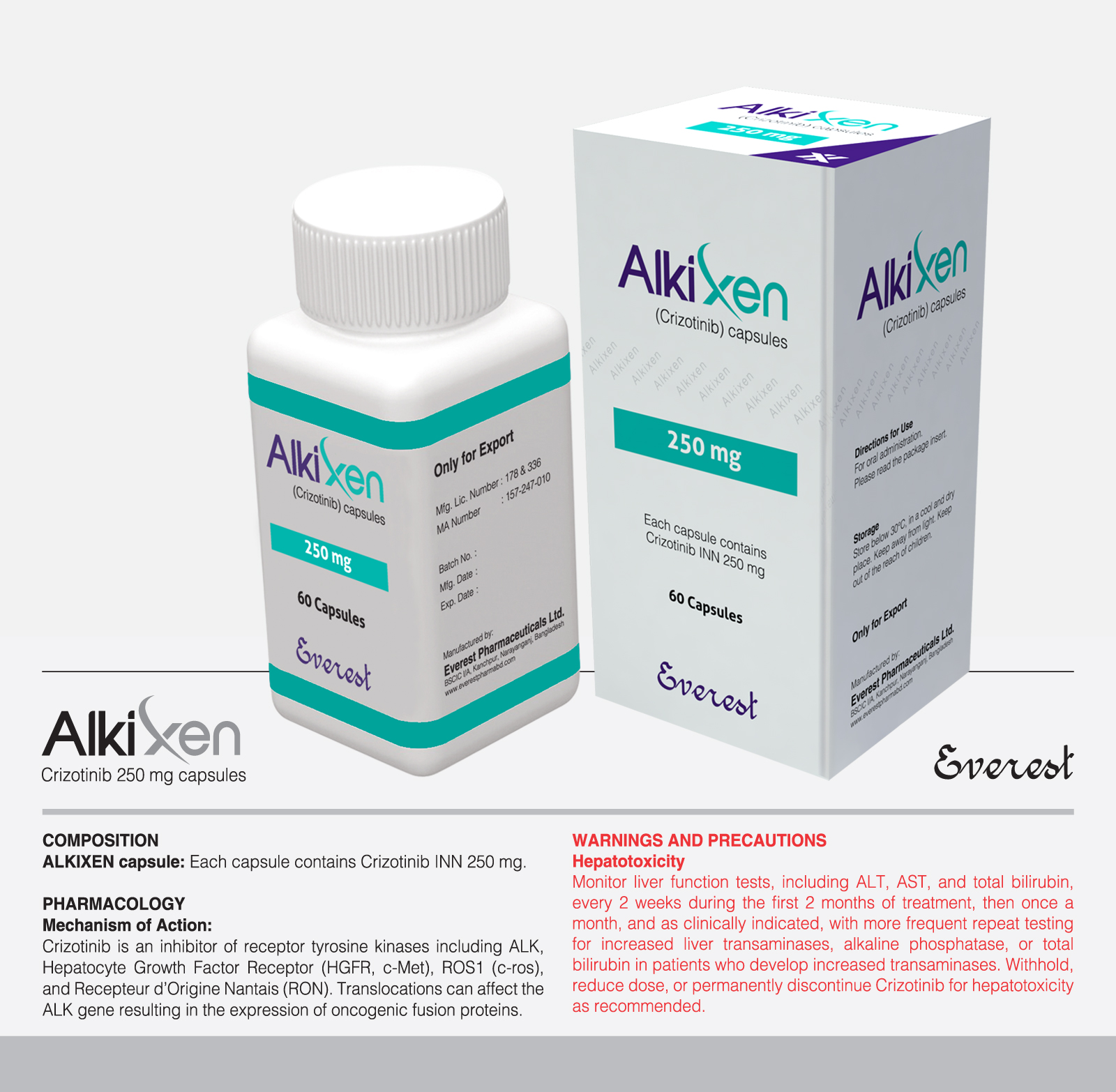
Tyrosine kinase inhibitor
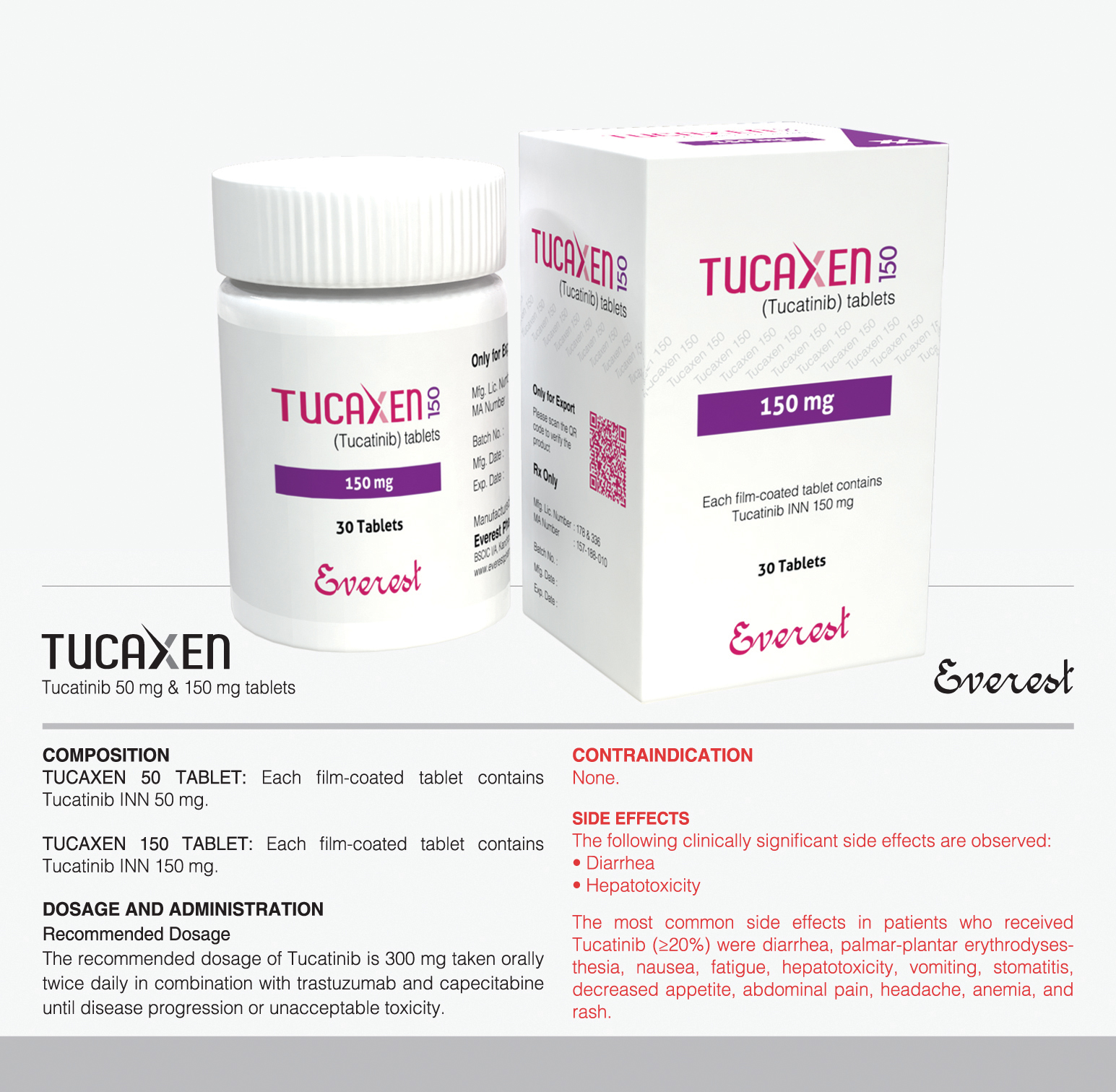
Tyrosine kinase inhibitor
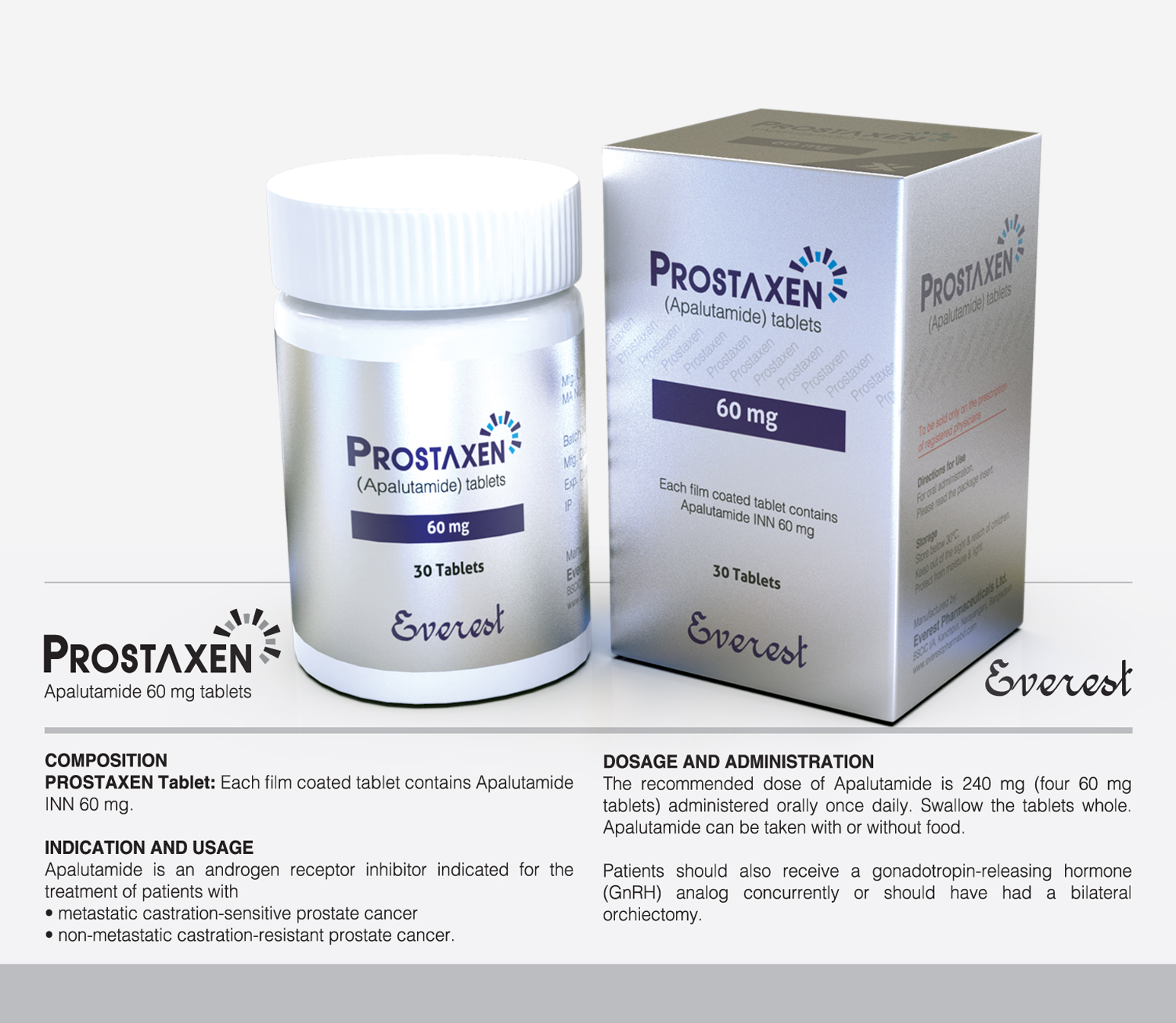
Androgen receptor inhibitors

Mezoxia

Tyrosine kinase inhibitor
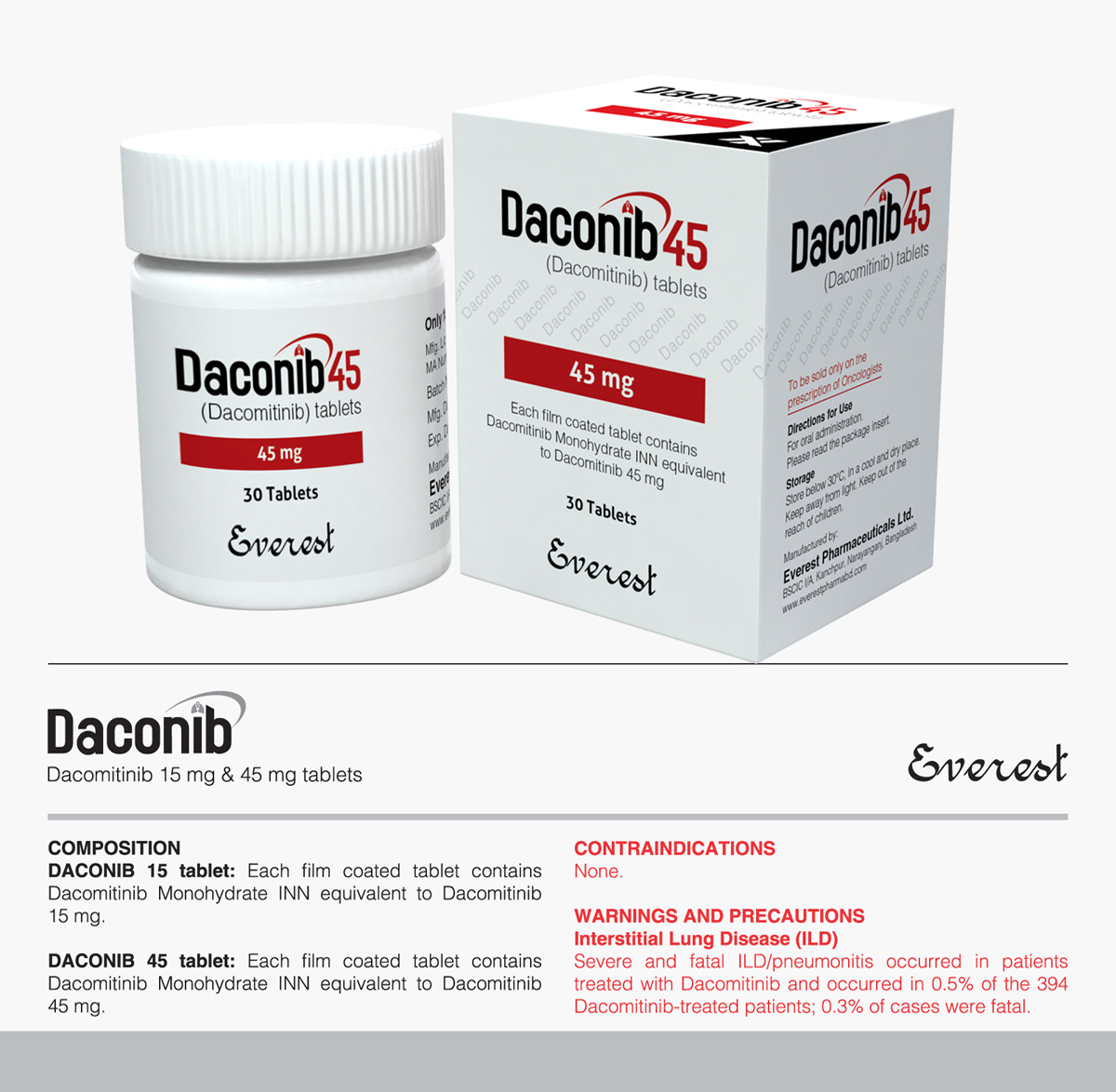
Tyrosin Kinase Inhibitor (TKI)

Tyrosine kinase inhibitor
Tyrosine kinase inhibitor
Tyrosine kinase inhibitor

KRAS inhibitors